






Concord: Lifesaving care for preterm babies improved by keeping the umbilical cord intact neonatal care, incubator, cord clamping
neonatal care, incubator, cord clamping
Concord: Lifesaving care for preterm babies improved by keeping the umbilical cord intact

Background:
Every year, 15 million infants are born preterm worldwide. Preterm birth is responsible for over 1 million deaths each year due to complications at birth, many survivors suffer from long-term disability, including learning problems, cerebral palsy or chronic lung problems.
Most preterm infants breathe insufficiently at birth, the cord is clamped immediately to not delay the respiratory support they need to survive. However, immediate cord clamping compromises the infants’ cardiovascular function, which can injure its immature organs. Waiting with cord clamping until the infant has been stabilized potentially reduces complications at birth, long term disabilities and mortality.
Technology Overview:
With Concord, delayed cord clamping for preterm babies requiring lifesaving care will now be a safe option. Concord is an innovative resuscitation table that makes it possible for the neonatologist to provide all care needed to stabilize the baby, while the umbilical cord remains intact. Concord has an adjustable support bed that can be positioned closely above the mother, on which the baby can be placed safely immediately after birth, to keep the sometimes very short umbilical cord intact. In addition, Concord keeps the baby close to the mother to allow bonding.
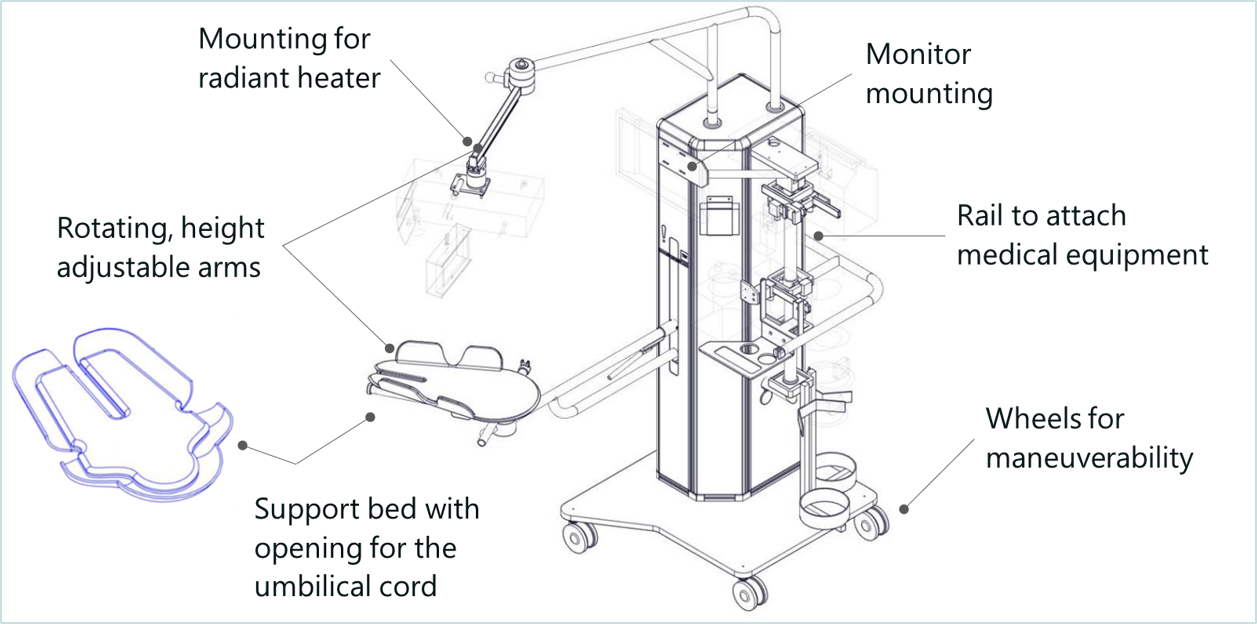
Benefits:
Many cord clamping studies compared immediate cord clamping with delayed cord clamping, focusing on breathing infants:
• Fewer babies needed blood transfusions for anemia – Relative Risk (RR) 0.61
• Reduced risk of bleeding in the brain (IVH) – RR 0.6
• Reduced risk of necrotizing enterocolitis (NEC) – RR 0.62
There is now data available showing another large benefit in delaying cord clamping until ventilation has been established. Results show that waiting with cord clamping until the lung has aerated and the infant has been stabilized leads to placental transfusion and a more stable oxygenation of the blood and a more stable heart rate during transition. This potentially decreases the injury to the infants immature organs, especially the brains and intestines. Concord therefore has the potential to:
• Reduce infant mortality;
• Reduce acute complications at birth;
• Reduce medical interventions;
• Less days in neonatal care units;
• Reduce the risk of long term disability;
• Reduce the societal economic cost of preterm birth.
Further Details:
Concord Neonatal B.V. was founded in April 2017 as a spin-out from LUMC, for the development and global commercialization of Concord.
Potential Applications:
Concord offers a solution for all worldwide hospitals specializing in the care of preterm newborns, to improve childbirth care for all newborns requiring resuscitation.
State of Development:
Specialists at Leiden University Medical Center (LUMC) invented Concord and developed a clinical prototype. The prototype of Concord is used for validation of feasibility and safety in a Phase 1 clinical study. Today, 28 babies have been successfully delivered using Concord at LUMC, in the delivery room as well as in the operating room. The results are very promising regarding the feasibility and safety of the workflow, the quality of care for the baby and the very positive feedback from parents.
Concord Neonatal aims to launch a commercial product by the end of 2018. To achieve this goal, Concord Neonatal is looking for €750,000 in external investment in 3 tranches of €250,000 in 2018, 2019 and 2021.
A pre-targeting approach for liver radioembolisation radiation therapy, theranostics, radioembolisation, pre-targeting, interventional radiology, nuclear medicine, supramolecular chemistry, pharmaceutical industry, biotech/medical companies
radiation therapy, theranostics, radioembolisation, pre-targeting, interventional radiology, nuclear medicine, supramolecular chemistry, pharmaceutical industry, biotech/medical companies
A pre-targeting approach for liver radioembolisation

Background:
Radioembolisation is a local form of radiation-therapy that is increasingly used to treat primary liver tumours and metastases untreatable via surgery or chemotherapy. Currently, radioembolisation procedures are performed in two steps: 1) a scout-step which is used to identify (lung) shunting and to optimize dose using the non-therapeutic radiotracer 99m-technecium magroaggregate, and 2) therapeutic-step using rather costly microspheres containing therapeutic radio-isotopes (90-Ytrium or 166-Holmium).
The sequential steps are performed as two discrete procedures, usually separated because of logistical reasons by a period of two weeks. While the clinical benefit of this approach has been demonstrated, its high cost and the preclusion of procedure-related toxicity to healthy tissue e.g. lung remains a challenge. Even when using a scout scan, shunting occurs in 10% and results in the displacement of a fraction of the therapeutic microspheres outside of the diseased area, leading to ineffective dose distribution and serious adverse effects such as radiation pneumonitis. Therefore a cheaper therapeutic alternative that provides higher accuracy is needed.
Technology Overview:
Firstly, LUMC researchers have used supramolecular chemistry to develop a two-step pre-targeting approach which integrates the scout- and therapeutic-steps in a single procedure. As the therapeutic component specifically targets the diagnostic component, this prevents discrepancies in accumulation. Hence, the dose prediction becomes more accurate and the shunting issue is solved. Uniquely, the chemical interactions chosen favour complex formation within the liver, meaning that the technology is less prone to toxic side-effects due to lung shunting.
Secondly, the pre-targeting concept used creates flexibility in the therapeutic radioisotopes that can be used for the procedure. This means more easily produced radioisotopes can be used to help bring down the treatment cost. Further, the flexibility in the use of radioisotopes also allows for the creation of kit-based radioembolisation formulation that can be prepared in the hospital. With that the current therapeutic window of two weeks can be shortened to one of only hours.
Thirdly, the supramolecular chemistry used in this invention could also be adapted for use in chemoembolization approaches or for the subcutaneous needle-injection based delivery of a therapeutic dose to isolated lesions. Again, in both these indications the ability to verify the accuracy of the delivery process before administering the therapeutic component is key.
Benefits:
This technology provides a more accurate and cost effective alternative to the radioembolisation procedures currently available. The technology:
Can use a wide range of (therapeutic) radioisotopes (in addition to currently used radioisotopes), as well as chemotherapeutics, creating the potential for new markets to be explored;Improves clinical logistics by allowing one day treatments. This avoids the need for the currently used complex double procedure (scout- followed by therapeutic-procedure) spread over two weeks);Allows for kit-based radioembolisation formulations to be created and with that improves the logistics of the supply chain.
Opportunity:
We are looking for partner(s) to license the technology for commercialization to and/or to fund research into refinement and clinical translation of the technology.
Please note, header image is purely illustrative.
Source: Philip Hogeboom, NL - Wikimedia Commons - CC 3.0 Unported (CC BY 3.0)
Imaging agents that specifically target peripheral nerves surgery, image guided surgery, imaging, nerves, pharmaceutical industry, biotech/medical companies
surgery, image guided surgery, imaging, nerves, pharmaceutical industry, biotech/medical companies
Imaging agents that specifically target peripheral nerves

Background:
Damage to nerves is a common side effect of surgery that can result in loss of function. Improved visualization of nerves in the operating field, for example by using florescence nerve imaging agents, would help the surgeon avoid such accidental nerve injury. Ideally, such nerve tracers need to be specific for the peripheral nervous system with little or no cross reactivity with other tissues (adipose and central nervous tissue) as this may lead to unwanted side effects. To date there are no nerve tracers in clinical application.
Technology Overview:
Research at LUMC, funded by two grants from the European Research Council, has led to the development of a peptide-based imaging agent that specifically binds to peripheral nerves. The imaging agent targets specific markers on the membrane of myelinating Schwann cells and contains a detectable fluorescent imaging label. Consequently it is suitable for use in fluorescence-guided surgery application. The imaging potential of this agent has been validated in myelin producing cells, dorsal root ganglia cultures, and in the myelin sheaths found in peripheral nerves, both ex-vivo and in vivo.
https://www.lumc.nl/org/radiologie/research/MIIGI/imilab/grants/
http://cordis.europa.eu/project/rcn/104526_en.html
Benefits:
Fluorescence guided surgery could be used to prevent accidental nerve injury and to allow surgeons to preserve nerves during complex orthopaedic, cardiologic or oncologic interventions.
In addition, the imaging agents might also be of value in:
- the assessment of neuropathies that involve degeneration of myelin (e.g. Multiple Sclerosis)
- localization of nerves during nerve repairing surgery after trauma or plastic surgery
- evaluation of nerve tissue using immunohistochemistry (ex vivo application)
- protein detection methodologies in research (e.g. western blot, ELISA)
Opportunity:
We are looking for partner(s) to license the technology to and/or to fund research into refinement and clinical translation of the technology.
Please note, header image is purely illustrative.
Source: OpenStax Anatomy and Physiology - Wikimedia Commons - 4.0 International (CC BY 4.0)
DNA-Probe for Non-Destructive Chromatin Sequence Extraction (nodeChrose) human genetics, chromatin, purification, DNA, LNA, nucleic acid, DNA probe, oligonucleotide, single-molecule, sequencing
human genetics, chromatin, purification, DNA, LNA, nucleic acid, DNA probe, oligonucleotide, single-molecule, sequencing
DNA-Probe for Non-Destructive Chromatin Sequence Extraction (nodeChrose)
Background:
DNA in eukaryotic cells is folded into chromatin, i.e. every 200 base pairs of DNA wrap around a core of histone proteins forming nucleosomes. Both the composition and the location of the nucleosomes play decisive roles in determining the organization of the whole chromatin complex. Moreover, differences in the regulation of the genes encoded in the DNA have been attributed to different chromatin configurations, giving organisms a means to activate specific sets of genes producing a selected set of proteins in different organs, while maintaining identical DNA copies in all cells. In fact, any intervention in the regulation of transcription, including activation/silencing of genes, involves not just bare DNA, but the complex of DNA and histone proteins.
Nucleosomes form a highly variant class. Their specific variable features include their positioning on DNA along the double-helix, and the occurrence of a number of post-translational modifications on DNA and histones. The occurrence of post-translational modifications is highly regulated and different characters are found in different organs. If fact, misregulation of post-translational modifications can be the origin of (epigenetic) diseases that can even be transferred from generation to generation.
The treatment of the (epi-)genetic diseases might greatly benefit from the capability of monitoring and/or influencing the positions and modifications of histones in chromatin. To avail of selected chromatin fragments extracted from the cell with their intact histone endowment and chromatin structure, is one key to the success of epigenetics research.
Technology Overview:
This technology can select and “pull down” sequence-specific chromatin fragments in a non-destructive way. This allows for highly focused analysis. i.e. zooming in on a single gene, of DNA sequences with their intact histone protein endowment.
At the core of this technology is a novel DNA-probe oligomer formulation, and a methodology to use it for efficient non-destructive chromatin sequence extraction (nodeChrose). This formulation returns a high-affinity probe which is specific to chromatin fragments embedding a known DNA sequence, the “target”, which is long enough to be unique in the genome.
In more detail, the probe is an especially designed oligonucleotide with a target-binding sequence at one end, in which some bases are LNA nucleotides (see “further details”, point (a) ), and at the other end is a covalently bound biotin, which selectively binds to streptavidin coated magnetic beads, allowing an easy pull-down of the extracted chromatin fragments (see “further details”, point (b) ).
The extraction process is initiated by the action of suitable restriction enzymes which selectively cut the chromatin chain and expose a short single stranded DNA portion, the “toehold”, where the oligonucleotide probe can at first “land” and bind. Thereafter, a “strand invasion” occurs at the cleaved end of the chromatin fragment (see figure) allowed by the transient opening of the double stranded DNA and promoted by the high affinity of the LNA-modified nucleotides at the oligonucleotide’s end.
Most remarkably, the whole nodeChrose process happens at room temperature. Such conditions are permissive for the purification of DNA-protein complexes under “native conditions”, where protein-DNA complexes are interacting as they are within a living cell, without the need for crosslinking agents, and without damage to the chromatin fragment nucleosome structure.
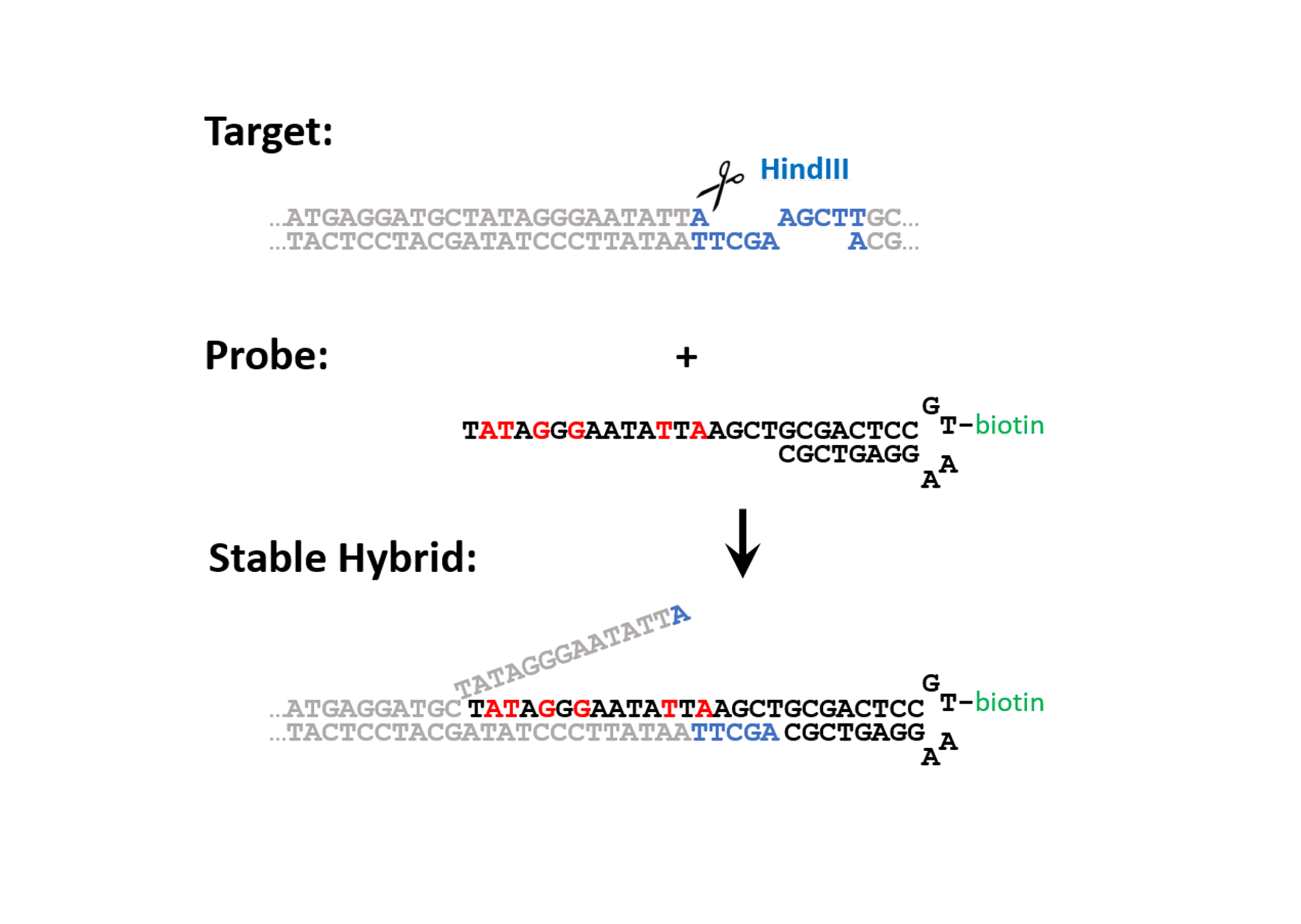
Figure 1: Design of the probe and generalized mechanism for the invasion of the probe into the target. The target is cut with a restriction enzyme creating DNA-toehold of 4 unpaired nucleotides. The probe consists of an 18 base pair overhang (complementary to the target sequence), a DNA hairpin where a modified base can be incorporated (e.g. a biotin), and a stacking sequence that caps the open end of the target. To increase the affinity of the overhang of the probe to the target sequence contains six LNA bases (colored in red). The mechanism of strand invasion can be summarized in four steps: 1) An endonuclease cleaves the target sequence such that a toehold appears. 2) The probe binds the target at the toehold. 3) Fraying of the double stranded target DNA adjacent to the probe drives strand invasion. 4) A stable hybrid between the probe and the target is acquired, which can be further purified by affinity purification.
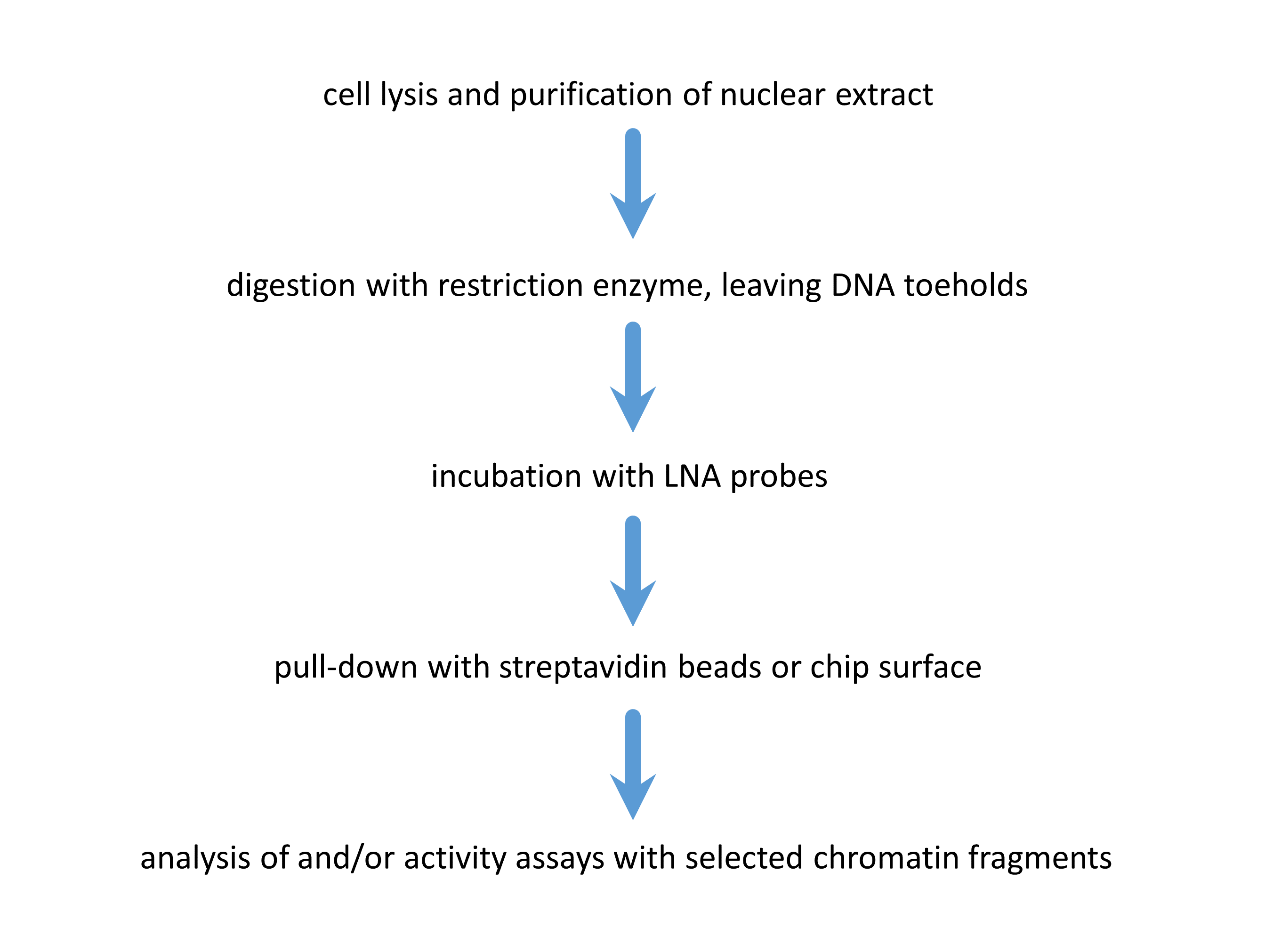
Figure 2: Experimental flow. First, the sample containing the DNA of interest is cut with a restriction enzyme, creating the toehold. Subsequently, the sequence specific probe containing the ligand for immobilization is added, so probe-target hybridization can occur. After this, the target can be pulled down with magnetic beads, or alternatively, immobilized on a surface for further analysis.
Benefits:
This technique overcomes most drawbacks of the other available methods for the extraction of nucleic acids, in that the latter employ any of the following:
- The use of high temperatures (80°C) to separate the two DNA strands and to allow oligonucleotide probes to clasp a target DNA sequence. While efficient, these methods denature completely the histone proteins and dissociate them from the DNA, with a total loss of all epigenetic features.
- The artificial creation of nucleosome structures in extracted DNA sequences. Although useful under many respects, these methods do not provide any specific epigenetic information, in that the obtained artificial chromatin does not reflect the chromatin in the living cells.
- Chemical cross-linking. This keeps the proteins attached to each other and the DNA, but inhibits further activity of analysis as the proteins are chemically different/non-functional. Moreover, chemical crosslinking is known to introduce artefacts.
- Bulk, averaged analysis, which obviously hides variations between chromatin compositions.
Further Details:
(a) Locked nucleic acids (LNA) residues are incorporated to increase the affinity of the probe for the target. LNA nucleotides are modified RNA nucleotides, with an extra covalent bond between the 2' oxygen and the 4' carbon. This modification results in a greater stability of the conformation of the sugar that favours hybridization, and results in higher melting temperatures for duplexes containing LNA bases.
(b) A sequence-specific purification of nucleic acids can be performed with a probe containing a biotin and magnetic beads coated with streptavidin. Furthermore, the target DNA molecule can be linked to two probes, one on each side, to increase specificity. A purification of the target can be done by, e.g., a pull down with magnetic beads, and/or by immobilisation on a surface of proteins with high affinity with the ligand attached to the probe (e.g. streptavidin).
Potential Applications:
This method can be applied in the field of protein research, as well as capturing DNA with a chip based approach in high throughput.
Single molecule analysis of native chromatin, as allowed by this technology, would be particularly useful for:
- any scientific research addressing epigenetics: it is highly cost-effective and has unprecedented resolution
- diagnosis: what does the chromatin landscape look like for a particular gene? Variations in chromatin composition have been linked to a variety of diseases.
- drug lead discovery in the field of epigenetics: once able to purify native chromatin fragments, one has the proper substrate for epigenetic enhancer/silencing factors. These could be used to identify compounds that interfere with such tasks.
State of Development:
This technology was originally developed to enable the use of single-molecule Force Spectroscopy on specific fragments of folded DNA/Histone complexes (chromatin), and as such it was already successfully employed, yielding insight on chromatin’s protein content and characteristics (this is reported in the upcoming scientific publication).
Opportunity:
This technology is already available for exclusive and non-exclusive licensing for commercial use, or for evaluation. However, should relevant opportunities for co-development present themselves, they would be surely taken into consideration, too.
RNA-targeted therapy for von Willebrand Disease small interfering RNA, inherited bleeding disorder, von Willebrand’s factor, thrombocytopenia, bleeding, RNA-targeted therapy, immunology
small interfering RNA, inherited bleeding disorder, von Willebrand’s factor, thrombocytopenia, bleeding, RNA-targeted therapy, immunology
RNA-targeted therapy for von Willebrand Disease
Background:
Von Willebrand disease (VWD) is the most common inherited bleeding disorder caused by qualitative or quantitative defects of von Willebrand factor (VWF). VWD has a prevalence of about 1 in 10,000 for patients with clinically relevant bleeding. Patients mainly suffer from mucocutaneous, post-traumatic or surgical bleeding, the more severe forms are associated with joint bleeds, thrombocytopenia or vascular malformations like gastrointestinal angiodysplasia.
Current treatment of VWD is focussed on increasing VWF plasma levels through administration of desmopressin or VWF-containing concentrates. However, in multiple situations these treatment strategies lack efficiency, since the production of mutant VWF is not dealt with. For example, the unhindered presence of mutant VWF may have negative effects like thrombocytopenia or the development of intestinal angiodysplasia. As most VWD (more than 90 per cent) is caused by dominant-negative missense mutations in VWF, a LUMC researcher hypothesized that the clinical phenotype may be ameliorated by diminishing expression of the mutant VWF allele.
Technology Overview:
The researcher has developed an approach and proved the principle of allele-specific inhibition of the mutant VWF allele by applying small interfering RNAs (siRNAs) targeting common single nucleotide polymorphisms (SNPs) in VWF. With a small set of SNPs, they are able to target a high percentage of patients, which would not be possible if each of the many identified VWD-related mutations is targeted.
- Efficient and allele-specific siRNAs against common SNPs in VWF have been selected
- Proof-of-principle for in vitro correction of a severe phenotypic (multimerization) defect has been shown
- Based on the frequency of the SNPs in the Caucasian population this siRNA approach may be applicable in over 75% of the VWD population
Further Details:
To be published very soon in peer-reviewed journal.
Liposome drug delivery vector targeting the blood brain barrier pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
Liposome drug delivery vector targeting the blood brain barrier
Technology Overview:
Researchers at Leiden University have developed a novel lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle with a selectivity for the brain endothelium (the blood brain barrier or BBB) of >10-fold over the systemic endothelium. This means that not <1% percent of the injected dose gets delivered to the brain and/or BBB, as is now the case with doxorubicin-filled liposomes, but potentially a 10-fold selectivity for brain endothelium over systemic endothelium or more of a drug can be delivered directly to the brain and/or BBB.
Potential Applications:
Drugs specifically targeting the brain and/or the BBB, such as treatments for strokes, cancer and neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, Huntington’s).Enhancement of brain and/or BBB (theranostic) imaging.
State of Development:
The BBB-selectivity was shown in zebrafish, that have a genome which is 70% homologous to humans and show a very similar brain morphology, organization and expression of key markers for BBB-function and integrity. Experiments in mammalian models are currently being undertaken.
The researchers have also demonstrated proof-of-principle of successful encapsulation of small molecule drugs as well as larger cargoes in the newly developed nanocarrier.
Method for generating PRRS vaccination strains arterivirus, PRRSV, swine, pig, vaccine, frameshift, antivirals
arterivirus, PRRSV, swine, pig, vaccine, frameshift, antivirals
Method for generating PRRS vaccination strains
Background:
Porcine reproductive and respiratory syndrome (PRRS) is the leading threat to the swine industry worldwide. Live-attenuated and inactivated vaccines are now commercially available, but are not without limitations, including concerns on reversion to virulence and insufficient level of protection. The co-existence of different PRRSV strains and subtypes emphasizes the need for cross-protective vaccines.
PRRS is the result of infection with a small, enveloped virus (PRRSV) containing a single positive-stranded RNA genome that can be divided into 2 major genotypes: Type I (European) and Type II (North American). Highly pathogenic variants that emerged in China and other Asian countries originated from the Type II genotype.
Technology Overview:
The PRRSV genome is about 15kb in length and contains at least 10 open reading frames. Situated in the 5’-proximal region of the genome are the PRRSV replicase genes, ORF1a and ORF1b, which represent nearly 75% of the viral genome. These replicase genes encode long polyproteins that are proteolytically processed into at least 14 nonstructural protein (nsp) products, the largest of which is nsp2. The invention provides the discovery and characterization of arterivirus protein, nsp2TF, the expression of which is dependent upon -2 ribosomal frameshifting at a site located in the nsp2 coding region. This coding region overlaps the portion of ORF1a that encodes the transmembrane region of nsp2 in PRRS and other arteriviruses, including lactate dehydrogenase-elevating virus (LDV) and simian haemorrhagic fever virus (SHFV). Mutations affecting the expression of nsp2TF impair PRRSV replication and result in a smaller plaque phenotype.
Provided here are arteriviruses that display reduced translation of nsp2TF and/or altered translation of one or more downstream products, arteriviruses in which nsp2TF function is reduced and/or absent, and vaccines or immunogenic compositions that comprise these arteriviruses. Also provided are diagnostic methods, methods for identifying compounds that inhibit -2 frameshifting, and gene expression tools for eukaryotic systems utilizing -2 frameshifting.
Benefits:
Potential improvements to the safety and/or efficacy characteristics of live PRRS vaccine such as quicker onset of immunity, broader cross-protection against virulent genetically diverse subtypes, reduced viral shedding to non-vaccinates and reduced persistence in vaccinated animals.
Potential Markets:
PRRSV infection in swine is characterized by later term reproductive failure in sows and severe pneumonia in neonatal pigs, and is the most economically significant disease of swine worldwide for the last 25 years. The annual worldwide impact is estimated at over $1 billion. Only a few countries with a representative population remain PRRS-free; the rest of the world is positive and suffers continual reinfections. A recent survey of UK vets estimated the prevalence of PRRS at approximately 50% of all sows and piglets. Improved antiviral therapies for PRRS are necessary.
A direct, high throughput assay for Neutrophil extracellular traps (NETs) pharmaceutical industry, biotech/biomedical companies, autoimmune diseases
pharmaceutical industry, biotech/biomedical companies, autoimmune diseases
A direct, high throughput assay for Neutrophil extracellular traps (NETs)

Background:
Neutrophil extracellular traps (NETs) are immunogenic, extracellular DNA structures that harness important auto-antigens to be recognized by the adaptive immune system. Recent evidence suggests that NETs have a role in a number of noninfectious diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), ANCA-associated vasculitis (AAV), diabetes, atherosclerosis and cancer. However, it is still unclear how and if NETs act as a common pathway in the pathophysiology of these clinically divergent autoimmune diseases. The exact role of NETs in these diseases remains to be elucidated and one limiting factor has been the lack of a well-defined assay to quantify NET formation. NETs are thought to play a role in the initiation of many noninfectious conditions, and, in combination with imaging NET production, this opens up the possibility of new therapies.
Technology Overview:
Researchers at LUMC have developed a direct, high throughput assay to quantifying NETs and are using this assay to study SLE and AAV patients. The assay directly visualizes and quantifies the amount of NETs produced on any given stimulus. The group at LUMC has a particular interest in autoimmune diseases, such as AAV and SLE). Any autoimmune diseases consist of periods of remission followed by episodes of disease activity (more about research and groups expertise can be found at http://www.einthovenlaboratory.com).
Benefits:
As the assay combines immunohistochemistry with quantification of extracellular DNA it provides an accurate assay that can be scaled up for high throughput.
Potential applications
This assay could be used as
- Diagnostic/ predictive test
- Clinical test of disease activity
- Ex vivo test for screening potential drugs
Further background:
http://www.nature.com
Novel TGF Beta modulator in Osteoarthritis TGF beta, osteoarthritis, biomarker
TGF beta, osteoarthritis, biomarker
Novel TGF Beta modulator in Osteoarthritis
Background:
Osteoarthritis (OA) is a painful and disabling condition of the joints affecting millions of people. Ageing is the primary risk factor, but how ageing results in OA is still an enigma. OA is characterized by degeneration of the articular cartilage, which has a very limited reparative capacity. Therefore, detection of early and minimum tissue damage is essential to stop the progression of the disease. However, diagnostic tools have low sensitivity and specificity and currently, there is no cure for OA.
Technology Overview:
To find novel druggable targets that modulate the pathological TGFβ signaling pathway in OA, researchers at the LUMC analyzed secreted TGF-β/BMP signaling modulators that are not essential for signaling but can potentiate or inhibit TGF-β/BMP signaling in cell/tissue-type and dynamic manner. They examined this in two OA mouse models that develop spontaneous OA during ageing, and in an inducible model, in which the medial meniscus is destabilized (DMM). Among others, they found a novel candidate that had not been linked previously to OA. They found that this novel modulator was expressed in mouse and human OA samples. In addition, they observed a correlation between its expression and the disease severity (Mankin score) in human specimens.
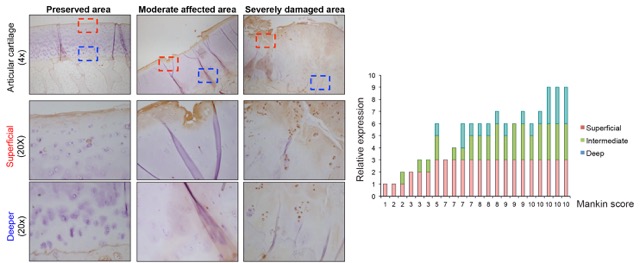
Figure 1: Immunohistochemistry analysis of human OA samples using a specific antibody against TGF modulator. Samples were scored for the expression of specific staining in chondrocytes of different areas of the cartilage and correlated to the severity (Mankin score).
_________________________________________________________
Preliminary in vitro experiments point out towards an involvement of this TGFβ modulator in chondrogenic differentiation processes. The scientists have found that its endogenous expression (mRNA and protein) was increased during differentiation of ATDC5 cells under chondrogenic conditions. Modulation of its expression (overexpression or knock-down) interfered with the terminal differentiation by respectively, increasing or inhibiting this process. Therefore, targeting such TGFβ modulator appears to be an intriguing option to foster TGFβ towards its anabolic profile, ceasing OA-progression.
 Figure 2: Modulation of the expression of the TGF modulator alters terminal differentiation of ATDC5 cells. ATDC5 stable cells with knockdown expression or overexpressing the candidate gene were seeded in micromass and hypertrophic differentiation was assessed by alizarin red staining. RNA was isolated from these cells and the relative gene expression versus GAPDH is shown in the graphs.
Figure 2: Modulation of the expression of the TGF modulator alters terminal differentiation of ATDC5 cells. ATDC5 stable cells with knockdown expression or overexpressing the candidate gene were seeded in micromass and hypertrophic differentiation was assessed by alizarin red staining. RNA was isolated from these cells and the relative gene expression versus GAPDH is shown in the graphs.
Mouse models of spontaneous thrombosis animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
Mouse models of spontaneous thrombosis
Background:
Thrombosis comes in two flavours:
- VT: Venous thrombosis (with pulmonary embolism as possible results)
- AT: Arterial thrombosis (with myocardial infarction or stroke as possible result)
Venous and arterial thrombosis are a major source of morbidity and mortality worldwide and both are complex vascular diseases for which pathogenesis is incompletely understood. Animal models are fundamental in our effort to understand the disease and develop better therapy (“Holy Grail” an antithrombotic without bleeding risk as side-effect).
Currently there are limited (venous thrombosis) or no (arterial thrombosis) technically reproducible or clinically relevant mouse models for these diseases.
Technology Overview:
Researchers at the LUMC have developed a mouse model for VT via “humanizing” mouse coagulation via RNAi of the hepatic antithrombin (Serpinc1) and protein C (Proc) genes.
In addition, they have developed a mouse model for AT via RNAi of Proc in Apoe-/- mice. In initial studies organized and large thrombi superimposed on an aortic root atherosclerotic plaque were observed, a unique and novel finding. This model needs further optimization.
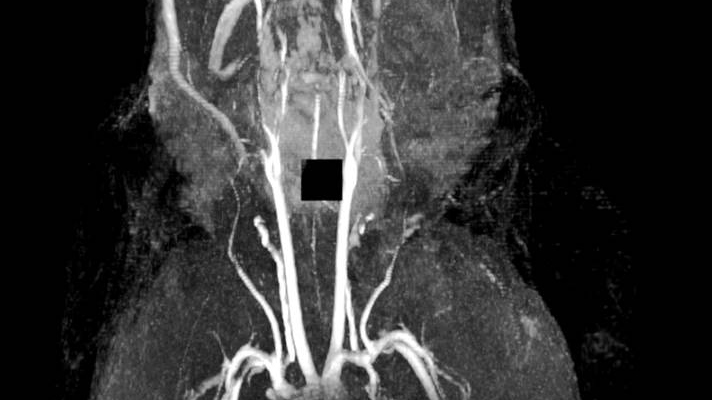
Figure 1: MRI of spontaneous venous thrombosis in a large vessel in the head (mandibular area)
Benefits:
The VTE model:
- Generates highly reproducible acute venous thrombosis
- Is technically simple and fast
- Reproduces morphologically venous thrombosis in humans
- Is responsive to (pharmacological) thrombin and platelet inhibition
- Is used by LUMC researchers and others to study VT pathogenesis
The AT model:
- Is technically simple and fast (i.e. the thrombosis part)
- Is reproducible, but has a low incidence (0.16)
- Responsiveness to drugs currently used to prevent AT unknown
- The occurrence of spontaneous atherothrombosis in the siProc apoE-/- mice is a truly unique event so far lacking in other preclinical models.
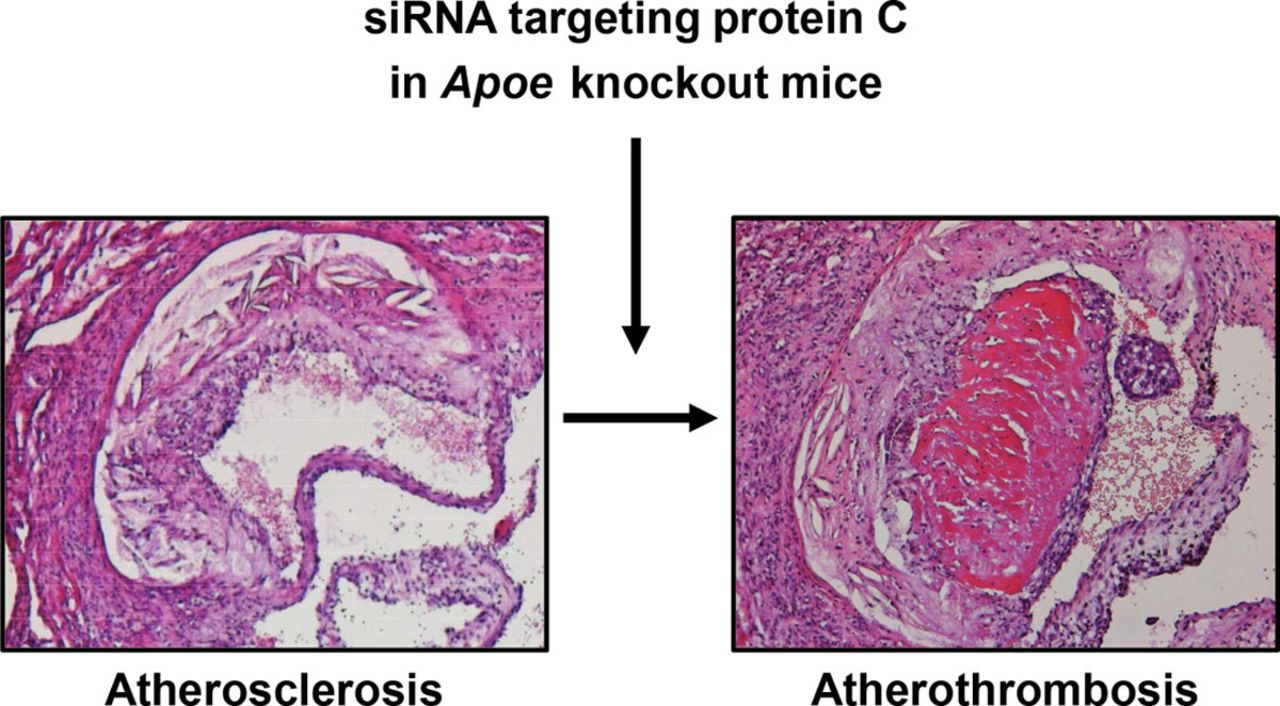
[Figure 2: Arterial (athero)thrombosis in apoliporotein E deficient mouse following RNAi]
Further Details:
VTE model: Heestermans et al., Blood. 2016 May 26;127(21):2630-7
AT model: Ouweneel et al., Arterioscler Thromb Vasc Biol. 2017 May;37(5):782-785
Applications:
Potential applications would be in preclinical research of venous thrombosis and pulmonary embolism, and arterial thrombosis and myocardial infarction and stroke.
Venous Thrombosis and Pulmonary Embolism (source: World Thrombosis Day 2016)
- Every year, there are approximately 10 million cases of VTE worldwide
- In the U.S., there are 100,000 - 300,000 VTE-related deaths every year
- In Europe, there are 544,000 VTE-related deaths every year
- Up to 60 percent of VTE cases occur during or after hospitalization, making it a leading preventable cause of hospital death.
Arterial Thrombosis and Myocardial Infarction and Stroke (source Netherlands Heart Foundation)
- Every day over 100 related deaths in The Netherlands
- Every day over 1000 cases hospitalized in The NetherlandsOver 1 million cases in The Netherlands
Opportunity:
Know-how on the VTE model is available for partnering and/or licensing.
LUMC are seeking co-development partners to further optimize the AT model and/or study its response to drugs that are currently used in MI/stroke (lipid-lowering/antiplatelet drugs) and to those in current pipelines (PAR inhibitors, FXI inhibitors, others).
Improved superconductivity with periodic nano/micro patterning superconductors, transition temperature, nanopatterning, phononic structure, electronic structure, NMR, MRI
superconductors, transition temperature, nanopatterning, phononic structure, electronic structure, NMR, MRI
Improved superconductivity with periodic nano/micro patterning
Background:
Superconductors are materials that can conduct electricity without resistance. Their superconducting behaviour is observed at and below very low temperatures (Transition Temperature, TC), generally under cryogenic conditions; all economically relevant superconductors have to be cooled down far below the boiling point of liquid nitrogen. Conventional superconductors as NbTi, NbSn3, Nb, and Al are used in sensing applications and magnets for nuclear magnetic imaging instruments. However, the costs of cryogenic cooling make most technologies based on superconductors very expensive and hinders a more widespread application.
Researchers at Leiden University have invented a method to improve the superconductivity in these and other materials.
Technology Overview:
This technology is based on a new approach to engineer/improve superconducting materials: deliberate alterations of the mesoscale structure of the material are realized by using nano and microfabrication techniques, resulting into a controlled modification of the phononic and electronic structure of thin films. This allows the coupling of the electrons with the phonon modes at higher temperatures, with the consequent formation of Cooper pairs and the onset of supercurrents. The physics model that underlies this technology shows how such periodic structures have to be designed to best improve superconductivity.
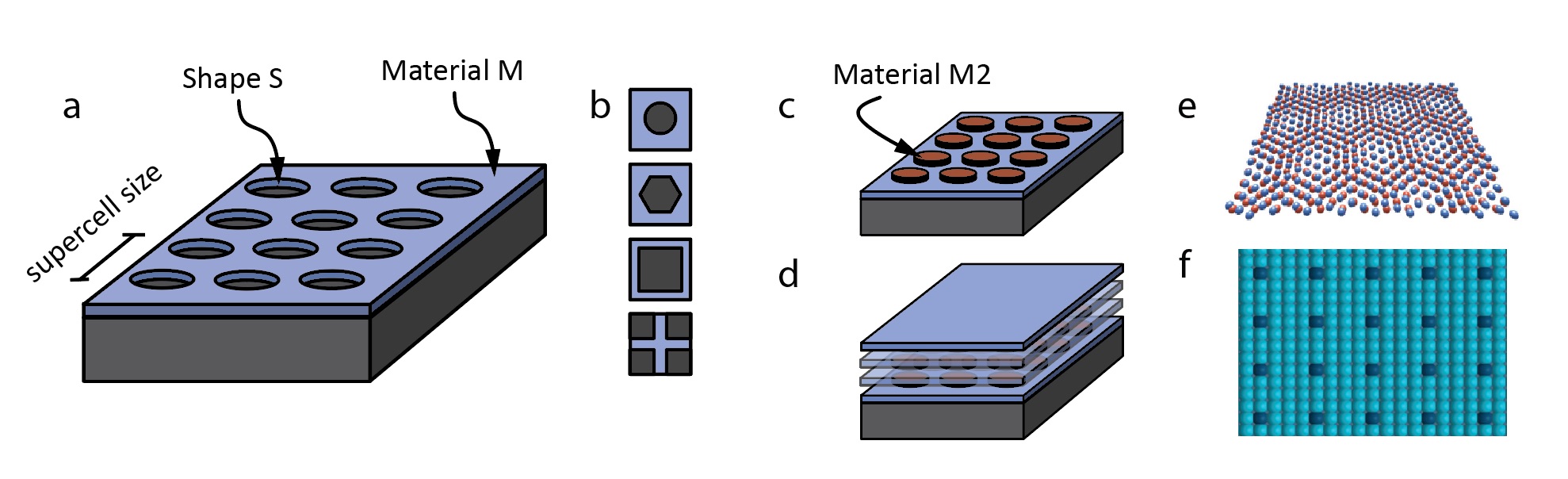
Figure 1: Possible fabrication methods and realizations. a. Modern nanofabrication tools allow to make periodic patterns. b. Different shapes are possible. c. Different layers of (insulating) materials on top of the thin films have different effects. d. Stacking allows for 3D materials. e,f. Smaller patterning are possible using Moire engineering or single atom manipulation.
Nanocapillary electrokinetic system for particle tracking / counting and microscopy particle count, nanoparticle, microparticle, particle tracking, flow cytometry, microparticle analysis, airborne particles, water analysis, vesicles, exosomes, colloids, liquid chromatography, microscopy, hollow fibre
particle count, nanoparticle, microparticle, particle tracking, flow cytometry, microparticle analysis, airborne particles, water analysis, vesicles, exosomes, colloids, liquid chromatography, microscopy, hollow fibre
Nanocapillary electrokinetic system for particle tracking / counting and microscopy
Technology Overview:
Tracking the motion of single nanoparticles in liquid solution is a gateway to high accuracy particle counting as well as to understanding and monitoring physical, chemical, and biological processes at the nanoscale. This technology has been recently demonstrated to carry out high-speed tracking of nanoparticles and macromolecules using elastic light scattering.
The weak scattering of single small viruses (26 nm) was successfully detected. For the first time, their fast thermal diffusion was tracked at a frame rate of more than 2 kHz (see Figure 1). As a step forward towards clinical applications, single urinary vesicles as small as 35 nm were also tracked by elastic light scattering (the first successful attempt of detecting biological vesicles that are smaller 70 nm in freely diffusing suspension). These vesicles possess low-refractive index (n<1.4), as confirmed by comparing their thermal diffusion and light scattering cross section.
 Figure 1: a. Schematic representation of our single nanoparticle/virus tracking setup. b. CCMV virus that we have tracked. c. The nanofluidic access of single-mode optical fiber. d. Quasi-1D tracking of a single CCMV virus.
Figure 1: a. Schematic representation of our single nanoparticle/virus tracking setup. b. CCMV virus that we have tracked. c. The nanofluidic access of single-mode optical fiber. d. Quasi-1D tracking of a single CCMV virus.
This particle-tracking system embeds a silica-based single-mode optical fibre with a hollow-core (nanometer scale) to suppress the free-diffusion of single nanoparticles and direct them into the detection volume. When light is coupled to the nanoparticle-filled optical fiber and detection is performed with a microscope lens at a right angle to the guided illuminating light, the untethered motion of nanoparticles can be imaged and tracked in a quasi-1D geometry for a virtually unlimited duration with negligible disturbance. For details see ACS Nano, 9 (12), 12349–12357, Open Access.
Benefits:
- Platform capable of detecting, tracking, counting, and measuring single nanoparticles, vesicles, and biomolecules.
- It uses a unique (patented) step-index hollow core fiber to propagate light without distorting the particle image (unlike other structured hollow core fibers).
- This method does not involve any nanofabrication processes, which makes it affordable to any research labs or diagnostic labs.
- Consisting of a plug and play cartridge along with a nanofluidic optical platform, the system does not require any modification of existing microscopy setup. Hence, it is a true add-on to standard optical microscopes.
- The platform will enable measurements at single nanoparticle level, electrophoretic separation, and studying diffusion and aggregation dynamics.
- This method does not require any fluorescent labels. Hence, it will be a label-free method.
 Figure 2: The portable stage setup. The green outlined portion is the fibre cartridge is magnetically attached to the stage setup. The cartridge is plug-and--play, which means it detachable or mountable without any mechanical tools. The red masked regions are part of a conventional optical microscope.
Figure 2: The portable stage setup. The green outlined portion is the fibre cartridge is magnetically attached to the stage setup. The cartridge is plug-and--play, which means it detachable or mountable without any mechanical tools. The red masked regions are part of a conventional optical microscope.
Potential Applications:
Ensemble scattering-based methods such as diffuse wave spectroscopy and dynamic light scattering spectroscopy have proven to meet the demand of a large market.
The current particle sizing methods are based on ensemble measurements. They are fundamentally prone to the uncertainties caused by inhomogeneous size distributions and stochastic phase-differences. Since dynamic processes are triggered stochastically, ensemble measurements cannot resolve fast processes that are washed out by averaging. The outcomes of such ensemble measurements are dominated by the slowest processes due to averaging of asynchronous processes.
One interesting and fast-growing field of application whereby this novel method can make a difference is the one of neurodegenerative diseases. The World Alzheimer Report of 2015 states that the current expenditure in the USA alone for dementia is USD 818 billion. By 2018 this cost will rise to USD 1 trillion. Currently, biochemical assays being used in any diagnostics are based on ensemble averaging experiments. However, neurodegenerative diseases are primarily caused by protein misfolding. The complexity of protein folding process is difficult or impossible to study using the available ensemble methods. Ensemble averaging experiments conceal molecular interactions, and thereby, early biomolecular interactions responsible for diseases, such as Alzheimer’s, Parkinson’s, prion diseases, and amyotrophic lateral sclerosis remain undetected. This limitation is overcome by this method, whereby the capability to track sub-micron particle individually is crucial to track the evolution of protein folding and misfolding.
Another area of biomedical research where particle tracking is in high demand is related to extracellular vesicles which are found in human body fluids. The scientific research on that is exponentially increasing every year (see Figure 3).
 Figure 3: An yearly exponential behaviour of number of scientific outcome (papers) on small biological extracellular vesicles (Edwin van der Pol, PhD Thesis, Amsterdam Medical Center).
Figure 3: An yearly exponential behaviour of number of scientific outcome (papers) on small biological extracellular vesicles (Edwin van der Pol, PhD Thesis, Amsterdam Medical Center).
Nanoparticle tracking has proven to be a viable technological market, too. This system can characterize nanoparticles from 10 nm to 2000 nm in solution, and allows for single particle tracking. However, trajectories being short, it gathers statistics by collecting many tracks, through which particle size distribution and concentration can be derived. This has an immediate impact on studying protein aggregation related neurodegenerative diseases. The primary advantage over existing alternative methods is that this system can resolve pure single nanoparticle level dynamics.
Prospective users of this technology can be from the following sectors:
- Biomolecular research
- Biomedical diagnostics
- Colloid chemistry
- Environment pollution
- Colloid chemistry
- Chemical identification
Opportunity:
This invention is a user friendly, plug-and-play add-on device for conventional optical microscope or method to tackle biologically relevant questions based on detecting single nanoparticles. The name of this device is ‘nanoCET’ (nano Capillary Electrophoretic Tracking) and encompasses a cartridge and a stage. Keeping the needs of the biomedical research into account, the nanoCET cartridge will be disposable and cost-effective, and can be detachable from the nanoCET stage. The nanoCET stage is an add-on the conventional microscope, which can be rented, leased, and purchased as one-time investment. Interested parties can perform test experiments in the inventor’s lab with the resident existing setups.
Atomically precise array of Nanopores in organic 2D membranes energy production, fuel cells, filter membranes
energy production, fuel cells, filter membranes
Atomically precise array of Nanopores in organic 2D membranes
Technology Overview:
Researchers at Leiden University have developed an entirely novel approach to the production of nanoporous graphene and other 2D atomic thin organic materials with nanopores in. The new technique has a number of major advantages over currently used lithography based techniques:
- it allows for large numbers of pores;
- it can produce small enough – sub nanometer – pores to match proton selectivity requirements for applications such as in fuel cells;
- it is possible to finely tune the rim of the pore.
The new technique particularly offers great potential for the production of nanoporous membranes for direct-methanol fuel cells (DMFCs) of which both durability and power density could be improved significantly using tailored 2D membranes. Tailored 2D nanoporous materials could also be used for water purification and many other applications.
Synthetic control on the tropism of cells as a means to enhance the efficacy of cell therapies cell therapy, cell imaging, biotech/medical companies,yropism, clinical, efficacy, synthetic control
cell therapy, cell imaging, biotech/medical companies,yropism, clinical, efficacy, synthetic control
Synthetic control on the tropism of cells as a means to enhance the efficacy of cell therapies

Background:
The degree of engraftment and infiltration of therapeutic cells is thought to be of high importance for the clinical efficacy of cell therapy. At the same time, low engraftment efficacy and survival of implanted cells are reported in clinical applications of cell therapy. In part the grafting can be optimized using local injections, but even then an adhesive strength is required to prevent loss via shunting or diffusion. This intervention provides synthetic control of the cell surface composition using fully reversible non-covalent surface modifications. This technique can be used to convert the cell-tropism for (diseased) cells and tissues of choice. At the same time the technology supports imaging based cell-tracking studies.
Technology Overview:
Using a pre-targeting approach, abundant membrane receptors on the therapeutic cells can be converted into binders of a multivalent cyclodextrin polymer. With that a homogeneous cell surface is generated that can be used to introduce a plurality of functionalities using host-guest chemistry. Such functionalities can entail e.g. an imaging label or a targeting vector. In case of the last, the affinity of a cell for other cells or tissues can be manipulated or enhanced.
Benefits:
While the use of cell-based therapies is growing, the clinical translation of transgenic cell modifying technologies is severely limited by regulations. The presented technology presents a generic chemical alterative that can be used to tailor the tropism of different existing cell types. With that market shares can be increased for available therapeutic cells. Since the technology is based on the membrane receptor expression of cells, it also provides a means to select cell populations out of heterogeneous cells samples of e.g. MsCs.
Potential Applications:
Cell therapies in humans are currently limited by the grafting affinity of cells, their viability and diagnostic methodologies that allow monitoring of these processes.
The invention provides a solution for these problems.
In the generation of 3D cell cultures it is of paramount importance that specific cell-cell interactions can be realized. The presented technology presents a means to artificially control such interactions.
Plasma biomarker for detection of onset of chronic Rheumatoid Arthritis Rheumatoid Arthritis, biomarker, plasma
Rheumatoid Arthritis, biomarker, plasma
Plasma biomarker for detection of onset of chronic Rheumatoid Arthritis
Background:
Rheumatoid Arthritis is characterized by inflammation of joints resulting in joint damage and disability. Research demonstrated that the presence of Rheumatoid Arthritis specific auto-antibodies directed against citrullinated proteins (ACPA) in the serum of patients increased the risk of developing the disease in patients with pain in their joints. ACPA can already be detected in patients years before onset of disease.
Technology Overview:
Researchers at the LUMC have recently discovered that ACPA isolated from Rheumatoid Arthritis patients is decorated with unique sugar structures. These sugar structures are not, or to a lesser extent, present on ACPA of people not yet diagnosed with Rheumatoid Arthritis. This unique sugar structure attached to ACPA could represent a biomarker of the transition phase from healthy to disease. Currently, researchers at the LUMC are investigating the possibility of a diagnostic test to analyse the sugar structures attached to ACPA on a large scale. Based on this test, the sugar structures of ACPA can predict the development of Rheumatoid Arthritis in patients with joint pain. This knowledge is important for clinicians to select an appropriate treatment in time to prevent progression towards chronic Rheumatoid Arthritis.
%2Bfigure%2B1.jpg) [Figure 1: ACPA with unique sugar structures are specifically present in patients with Rheumatoid Arthritis and might be crucial to predict progression from auto-antibody positive healthy subjects to patients with full-blown, chronic and persistent arthritis.]
[Figure 1: ACPA with unique sugar structures are specifically present in patients with Rheumatoid Arthritis and might be crucial to predict progression from auto-antibody positive healthy subjects to patients with full-blown, chronic and persistent arthritis.]
Benefits:
ACPA appear in the blood of Rheumatoid Arthritis patients up to ten years before onset of disease. Currently it is impossible to predict the exact time point of disease manifestation. Therefore, treatment starts at diagnosis of the disease. At this stage, the disease is already chronic, and the patient is devoted to lifelong treatment. This invention allows clinicians to intersect the healthy auto-reactive positive pre-disease phase from the pathogenic phase in which Rheumatoid Arthritis development commenced. If in this stage therapy is applied, development towards Rheumatoid Arthritis might be stopped before chronification occurs.
Further Details:
More background information can be found in the publication: “Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis.”, Rombouts et al., Ann Rheum Dis. 2016 Mar.
Potential Applications:
Identify ACPA+ individuals with high risk to develop Rheumatoid ArthritisTargeted treatment - distinguish individuals with high risk to develop Rheumatoid Arthritis in the “at risk” group and start treatmentEarlier start of treatment to prevent/delay/decrease severity and chronification of Rheumatoid Arthritis
Opportunity:
The researchers are looking for partner(s) to license and further (co-)develop and market this assay for clinical application. Specifically companies that are developing diagnostic tools for inflammatory diseases.
IP Status:
Priority patent filed
Reversible immortalization method for generation of homogenous, stable & authentic human cell lines pharmaceutical industry, biotech/medical companies, cardiomyocytes, immortalisaton
pharmaceutical industry, biotech/medical companies, cardiomyocytes, immortalisaton
Reversible immortalization method for generation of homogenous, stable & authentic human cell lines
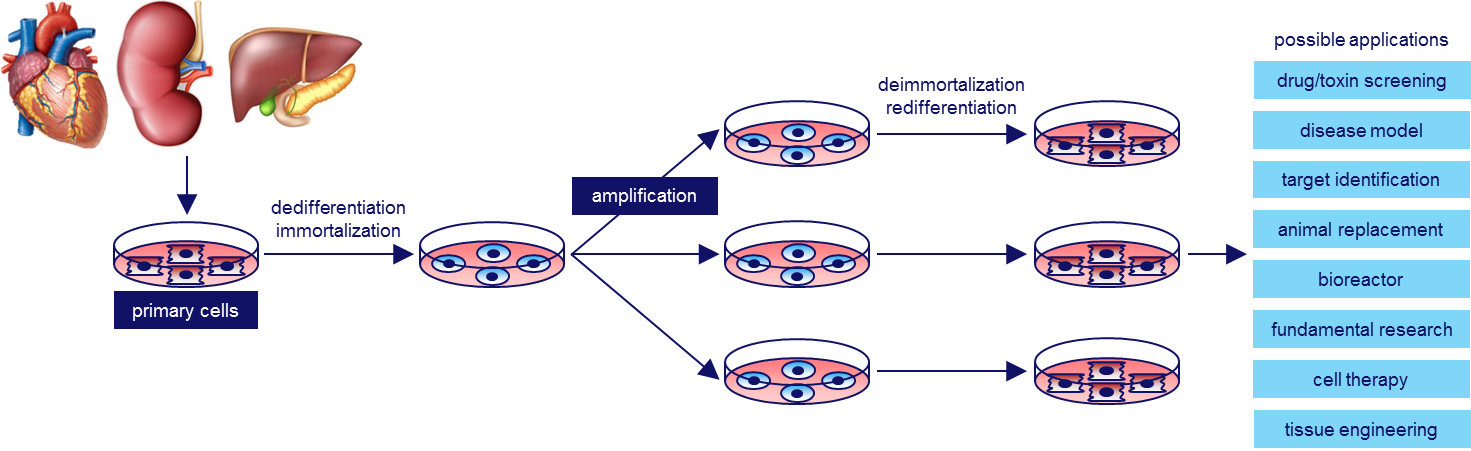
Researchers of Leiden University Medical Center have discovered a broadly applicable immortalization method for mammalian (including human) cells.
This method allows expansion and redifferentation of your cell type of choice through gene transfer and simple changes in culture medium composition.
One way to obtain large numbers of differentiated cells from small tissue samples (i.e. biopsies) is by permanently immortalizing the cells directly after isolation followed by their expansion in a dedifferentiated state and their redifferentiation using specific medium formulations. This, however, rarely yields cells in an advanced state of differentiation (i.e. authentic cells) due to the continued presence of proliferation stimuli.
This invention concerns the discovery of a new immortalization technique that overcomes many of the shortcomings of the existing immortalization strategies and allows the reproducible generation of large numbers of differentiated cells with very similar properties as the cells from which they have been derived. These differentiated cells may provide a superior alternative for cell-based systems relying on differentiation of, for instance, pluripotent (human) stem cells and could therefore become the platinum standard for cellular model systems (e.g. for drug testing) & production platforms (e.g. for 3D tissue printing or to produce biopharmaceuticals).
Treatment of familial blindness gene therapy, virology, orphan diseases, biopharmaceuticals, eye
gene therapy, virology, orphan diseases, biopharmaceuticals, eye
Treatment of familial blindness

An adeno-associated virus-based recombinant gene therapy vector for the treatment of a retinal disorder, specifically Leber's congenital amaurosis and retinitis pigmentosa, due to mutations in Crumbs homologue-1.
LCA and RP are rare genetic disorders, caused by mutation in the Crumbs homologue-1 (CRB-1) gene, with prevalences of 1/40,000 and 1/3,000 respectively.
Scientists from Leiden University Medical Center (LUMC) and the Netherlands Institute for Neuroscience of the Royal Netherlands Academy of Arts and Sciences (KNAW) have developed an adeno-associated virus (AAV)-based recombinant gene therapy vector for the treatment of a retinal disorder, specifically Leber's congenital amaurosis (LCA) and retinitis pigmentosa (RP), due to mutations in Crumbs homologue-1 (CRB-1). Presently there are no therapeutics or effective treatments available to prevent, delay or treat LCA or RP in humans. Therefore, there is a need for methods and means for the treatment of retinal disorders due to mutations in CRB1.
LUMC has attracted significant funding for the preclinical development and Phase I/IIa clinical trial. We are currently looking for a development partner to collaborate, contribute expertise and / or provide matching funds for the clinical development stage, in return for a license or exclusive option to license the know-how and global patent family.
This project is a unique opportunity for a public-private partnership to develop a treatment for a rare orphan disease.
The development of therapeutics for CADASIL patients CNS, CADASIL, gene therapy, rare disease, orphan, exon skipping, oligonucleotides
CNS, CADASIL, gene therapy, rare disease, orphan, exon skipping, oligonucleotides
The development of therapeutics for CADASIL patients

Scientists at Leiden University Medical Center (LUMC) developed a potential method for the therapeutic intervention in patients suffering from CADASIL.
CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is a condition causing ischemic brain lesions, which gradually leads to cognitive decline and eventually to dementia. Currently, there is no treatment.
The disease is caused by characteristic mutations in the NOTCH3 gene resulting in an unequal number of cysteine residues and misfolding of the NOTCH3 protein.
NOTCH3 is exclusively expressed in vascular smooth muscle cells (VSMC) and this misfolding leads to an accumulation of the extracellular domain of the NOTCH3 protein and granular osmiophilic material on the surface of degenerating VSMC. In turn, this leads to impaired vascular reactivity and decreased cerebral blood flow.
Scientists at LUMC have succeeded in re-establishing an equal number of cysteine residues in the NOTCH3 protein by the exclusion of specific exons from the mRNA. They demonstrated that this reduces or even delays the accumulation of NOTCH3 on the surface of VSMC. This novel finding could lead to the development of therapeutic strategies for CADASIL patients.
Partner companies are now sought for research collaborations in this field, and licensing of key technologies. Specifically we are looking for companies with a a franchise in the treatment of CNS-ischaemic diseases.
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank obesity, metabolomic disease, biobank
obesity, metabolomic disease, biobank
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank

Interested in conducting research on obesity and metabolic disease?
Leiden University Medical Center Netherlands has finished enrollment of the Epidemiology of Obesity Study (NEO). Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers in their pursuit of causes and treatments for obesity and metabolic disease. Information ranging from health and depression questionnaires to heart and brain MRIs has been collected from 6,000 participants with a BMI = 27 kg/m2 or higher and 1,000 participants with a BMI <27 kg/m2.
Endpoints include diagnosis of Type 2 diabetes, cardiovascular disease, COPD, asthma, chronic kidney disease, osteoarthritis and all-cause mortality. Analyses were conducted on blood, serum, urine and plasma. Serum, DNA, RNA have been saved for future studies.
The database/biobank is now open for access. LUMC investigators involved in the NEO Study are also interested in research collaborations using the database/biobank.
For more information please find the link to the abstract and full publication here:
Abstract: The Netherlands Epidemiology of Obesity (NEO) study
Publication: The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection
Device to remove unwanted solvents from biochemical samples analytical (bio)chemistry, liquid chromatography, NMR
analytical (bio)chemistry, liquid chromatography, NMR
Device to remove unwanted solvents from biochemical samples

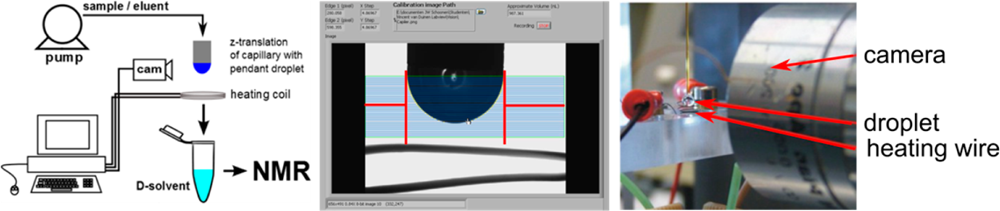
Scientists at Leiden University have invented a simple and robust approach to efficiently remove unwanted solvents from liquid mixtures containing dissolved chemical components.
Biochemical analysis of complex mixtures is of great importance in various fields of application. A combination of multiple analytical techniques, such as LC-MS, GC-MS and LC-NMR, is often needed to achieve sufficient molecular separation and enrichment. The combination of LC with NMR is not straightforward, certainly not for polar molecules. Scientists at Leiden University have developed an efficient and robust interface between LC and NMR to overcome this problem.
The exchange of solvent is achieved by the controlled evaporation of a (hanging) droplet using a machine vision feedback loop. The apparatus was succesfully tested for a multitude of samples containing volatile, thermosensitive, polar and nonpolar analytes.
Leiden University is looking for partners for further (joint) development of the device and is looking to license this powerful technology to commercial part(ies).
Capillary transfer of single droplets analytical (bio)chemistry, LC-NMR, sample transfer
analytical (bio)chemistry, LC-NMR, sample transfer
Capillary transfer of single droplets

Transferring samples from one capillary to a second capillary is not straightforward. Even more so for small quantities such as droplets.
Scientists of Leiden University have developed an apparatus to transfer droplets from a first capillary to a second capillary.
For sample handling and sample pre-treatment, the sample is flowing through small tubes in many applications. At Leiden University scientists had concentrated a sample in a droplet at the end of a capillary (Anal. Chem., 2013, 85 (12), pp 5734–5739). This droplet needed to be transferred to a second capillary for further handling of the droplet (e.g. transportation to NMR).
The system developed for this purpose has been build and tested successfully. The system is fully automated and has proven to be very robust. Leiden University is seeking commercial partners to (co)develop this into commercial products. Applications can be very broad, Leiden University has concentrated its efforts towards sample handling for LC-NMR.
A working set-up is available and so are experimental data using the set-up.
Power tool attachment to cut an oval shape in bone medical device, power tool, orthopedics, surgery, oncology
medical device, power tool, orthopedics, surgery, oncology
Power tool attachment to cut an oval shape in bone

Inventors at the Leiden University Medical Center have developed a power tool attachment that cuts an oval shape.
In order to treat chondrosarcoma by curettage, an opening in bone is needed which is most often accomplished by drilling four holes and connecting them. Two disadvantages of this method are: a) the creation of sharp, squared corners which are weak points that promote breakage of the bone, and b) the loss of bone from cutting holes. This new saw creates an oval opening in the femur. This has several advantages over the conventional method. Importantly, the oval shape retains the advantage of creating a shape with a length similar to a rectangle, thus maximizing the area of the shape, but with round edges. Other advantages are that the width of the cut is 0.1 – 0.2 mm, much less than standard, thus the healing time is shortened when the bone is replaced. Furthermore less skill is needed to cut a shape in bone with an oval saw than by conventional methods.
The current length of the oval shape is approximately 3 cm but the mechanism can be developed to cut an oval shape of a different size. A smaller cutout would allow access to tumors in smaller bones.
The bone saw may also be useful to create a bone flap for cranial surgery in which the bone will be replaced. Another application may be for harvesting bone. The attachment was designed to fit an existing hand-held power tool but can be adapted. The saw can cut multiple types of materials and therefore may be useful outside the medical device arena.
Single atom graphene nanogap biomolecule detection, sequencing, spectroscopy
biomolecule detection, sequencing, spectroscopy
Single atom graphene nanogap

Chemists at Leiden University have developed a method yielding a device separating two single carbon atoms from two individual conducting graphene layers.
The invention enables the fabrication of a graphene nanogap using a simple methodology. It has been impossible till now to fabricate a graphene nanogap.
The next stage of improvement includes (i) to define the (bio)chemical sensitivity of the device, (ii) chemical edge passivation and determination of the utility of the device as a spectroscope for graphene (and other 2D crystal edge characterization), (iii) to attempt the translocation and detection of individual (bio)(macro)molecules, and (iv) to design the micro/nanofluidic platform (for ‘controllably’ delivery of a single biomolecule to the point contact; for sequencing or characterization of molecules).
Plasma antenna for MRI imaging, medical diagnostics, MRI
imaging, medical diagnostics, MRI
Plasma antenna for MRI
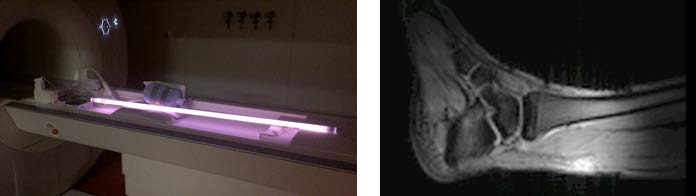
Scientists at Leiden University Medical Center have developed a plasma antenna to perform magnetic resonance spectroscopy and imaging.
The plasma antenna can be used to replace the conventional metal antenna and offers increased flexibility for MRI. The plasma can be turned on and off on the order of microseconds, and eliminates the requirement for decoupling transmit and receive MR coils. The plasma is reconfigurable and can be controlled by the input power. Using a plasma rather than metal eliminates attanuation from metal in simultaneous PET and MRI data acquisition.
Additional development is necessary to determine the most advantageous design and placement for the plasma antenna to be able to incorporate the antenna into existing MR machines and achieve a high signal to noise ratio.
We are seeking a licensee and development partner.
Novel automated sample purification and enrichment for DI-MS analytical (bio)chemistry, mass spectroscopy
analytical (bio)chemistry, mass spectroscopy
Novel automated sample purification and enrichment for DI-MS
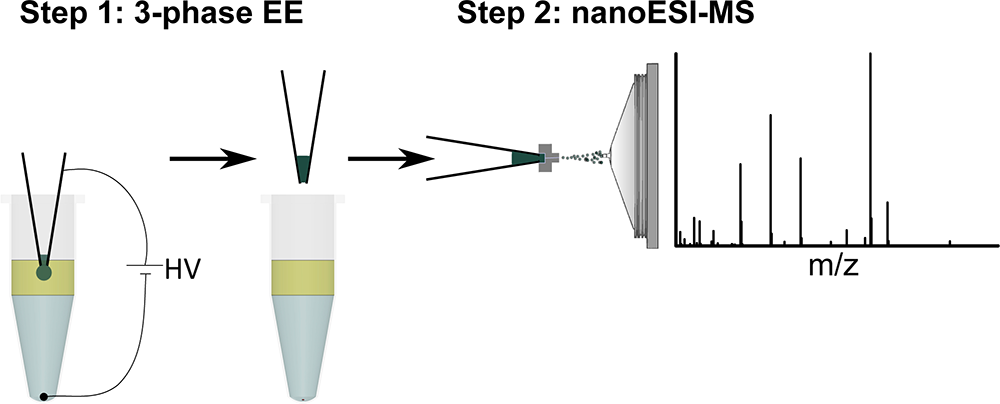
Scientists at Leiden University have invented a method for easy and automated (bio)sample preparation for DI-MS.
The method allows for easy and fast sample purification and enrichment and can easily be integrated with commercially available nanoESI robots.
The aim of Direct-Infusion Mass Spectrometry (DI-MS) is to provide compositional information as well as identification and quantification of specific analytes. Especially nanoelectrospray (nanoESI) is very powerful, but nanoESI emitters are susceptible to clogging due to e.g. protein precipitation and salt crystallization.
The new preparation method developed in Leiden is based on electroextraction and can be used to selectively extract cations or anions from a sample before infusion into the Mass Spectrometer. This way, for example, proteins and salts can be discarded and therefore the quantitative power of DI-MS can be significantly improved. The method also has the potential to pre-concentrate analytes before infusion into the MS. This entire method has the additional advantage of being easily integrateble with current (robotic) liquid handling/nanoESI systems.
Leiden University is looking for partners to bring this technology to the market.
Fabrication of graphene nanopore/nanogap structures with implemented nanofluidic channel biomolecule detection, sequencing, electron tomography
biomolecule detection, sequencing, electron tomography
Fabrication of graphene nanopore/nanogap structures with implemented nanofluidic channel

Chemists at Leiden University have developed a new technique for the fabrication of nanogap devices.
This technique which is considerably faster than conventional methods and requires low level of fabrication accuracy. It is a platform for the realization of biomolecule detection/sequencing and electron tomography.
The translocation of biological molecules have been detected successfully in nanopores, fabricated in two-dimensional (2D) materials. The mono-atomic thickness of 2D materials is comparable with the spacing between bases composing biomolecules, hence such materials can potentially provide enough resolution for single base identification. The fast translocation of biomolecules - which is not traceable monitoring the ionic current through the nanopore - is an important limitation for the development of nanopore systems for sequencing purposes.
Two graphene electrodes positioned very close to each other with a nanoscale gap in between is a model system to achieve biomolecule sequencing. The electrical current tunneling between the electrodes depends on the geometry and chemical composition of the bases traveling through the gap; fast enough for sequencing.
While conventional nanofabrication techniques have failed to realize nanogap devices in two-dimensional materials so far, the simplicity of our new fabrication technique promises fast development of the devices. High resolution and strong signal to noise ratios are predicted detecting biomolecule bases in nanogap schemes.
Dynamic re-usable RAMAN scattering sensor chemistry, sensors, (bio)chemical analysis
chemistry, sensors, (bio)chemical analysis
Dynamic re-usable RAMAN scattering sensor
Scientists at Leiden University have developed a novel Surface Enhanced Raman Spectroscopy (SERS) sensor which they have named SERSOR.
This SERS-based sensor allows for dynamical and re-usable measurements and can be used with standard RAMAN equipment.
Surface enhancement in RAMAN-spectroscopy has proven to greatly improve the RAMAN-signal, increasing sensitivity with several orders of magnitude. Unfortunately, until now, surface enhancement could not efficiently be integrated in most RAMAN-based sensor applications as surface enhancement technology is limited to one shot measurements due to irreversible binding of the analyte to the SERS-substrate. This makes SERS-technology not suitable for use in applications where continuous dynamic measurements are required.
Scientists at Leiden Univesity have developed SERS-based sensors that can make dynamic measurements of their environment over time. Sensors like these would be ideally suited for application in, for instance, industrial process monitoring. They can continuously monitor flows of substances (in aqueous solution) for contaminations or formed substances and upon identifying such substances, measure their concentration over time.
Furthermore, this new technology makes the SERS-substrates re-usable, which allows for calibration of experimental setups and greatly reduces the number of substrates required per experiment. This makes the new SERSOR very suitable for applications where many different molecules need to be analyzed.
Wide Field SpectroPolarimetry remote sensing, air quality measurements, optics
remote sensing, air quality measurements, optics
Wide Field SpectroPolarimetry
Scientists at Leiden University have invented a novel device to measure spectropolarimetry over a large field of view in one or two dimensions.
The device is free of moving parts and is suited for both earth based and space based measurements.
Measuring spectropolarimetry over large fields of view is difficult. The wide field optical elements introduce unwanted polarimetric effects. Additional and non-trivial calibration is needed and needs to be repeated, for example as the coatings on the optical elements age. Many devices also use moving parts to scan different directions making the instrument more complicated and more prone to failure.
Scientists at Leiden University's Observatory have invented a device that eliminates these limitations. Proof-of-principle has been reached and Leiden University is looking for partners to further develop a fully operational (commercial) device.
The scientists have been working on validating the technology for applications with respect to air quality monitoring. Many more applications are foreseeable and we are looking for partners that would benefit from adding (spectro) polarimetry measurements to their products.
Natural ionic liquids and deep eutectic solvents compound extraction, compound solubilization, natural products, drug delivery, biopharmaceuticals
compound extraction, compound solubilization, natural products, drug delivery, biopharmaceuticals
Natural ionic liquids and deep eutectic solvents

Researchers from Leiden University and colleagues from Delft have identified novel natural ionic liquids and deep eutectic solvents (NADES).
NADES may be used for highly efficient extraction and storage of natural products from plants, such as pharmaceuticals and bio-actives, flavours, natural colorants, etc. Since NADES consist of simple, cheap, and naturally occurring compounds with a high safety profile, extracts may directly be used in food, pharmaceutical, cosmetical and agrochemical applications.
Ionic liquids (IL) and deep eutectic solvents (DES) consist of two or more solid crystalline compounds that, when mixed together, form a liquid with unique properties. Currently, IL and DES consist of mostly toxic, bulky and asymmetric organic cations and are widely used as solvents for industrial processes such as organic synthesis and extractions.
Researchers from Leiden University and colleagues in Delft have developed a range of new NADES, that consist solely of natural compounds that are normally present in cells, such as certain sugars, simple organic acids and amino acids.
As a proof-of-principle, it was shown that NADES are excellent solvents for extraction of compounds from biological materials which are otherwise difficult to isolate. Examples include plant colorants and drugs, suggesting applicability in drug delivery and the discovery of novel bio-active plant compounds.
NADES can replace existing synthetic ILs and DES that contain toxic compounds and are thus difficult to dispose of. As the NADES constituents are naturally occurring and safe, very simple to make and cheap, many applications can be envisioned including drug delivery, and extraction of various compounds for use in food, cosmetics or pharmaceuticals.
Reduction of antibiotic resistance - Co-administration of food-grade compounds antibiotics, antibiotic resistance, antibiotic adjuvant
antibiotics, antibiotic resistance, antibiotic adjuvant
Reduction of antibiotic resistance - Co-administration of food-grade compounds

Scientists at Leiden University's Institute of Biology have found compounds that reduce antibiotic resistance in pathogenic bacteria.
In the absence of an antibiotic agent, such compounds have no/hardly any bactericidal effect. The compounds are food-grade. Co-administration together with existing drugs will potentiate their effect. This should allow us to re-use antibiotics that have now been abandoned to due to resistance problems. Currently, the scientists are seeking partnerships for the next stage of development.
The antibiotics market (over 40 billion USD) is hampered by the lack of new (approved) compounds to answer the growth of antibiotic resistance that poses an increasing threat to treat bacterial infections. Only two completely new classes of antibiotics have been introduced over the past 30 years: the oxazolidinone linezolid (Zyvox; Pfizer) in 2000 and the cyclic lipopeptide daptomycin (Cubicin; Cubist) in 2003. Current approaches are focussing on pro-drug strategies, species-specific platforms (identification of (new) specific targets/pathways), and mining untapped sources of natural compounds.
This current invention allows potentiating existing antibiotics by counteracting antibiotic resistance. While the activity of the compounds is broad, we have established their positive effect in particular for enhancing the efficacy of aminoglycosides and β-lactam antibiotics. The enablement is further helped by the fact that the compounds are food-grade.
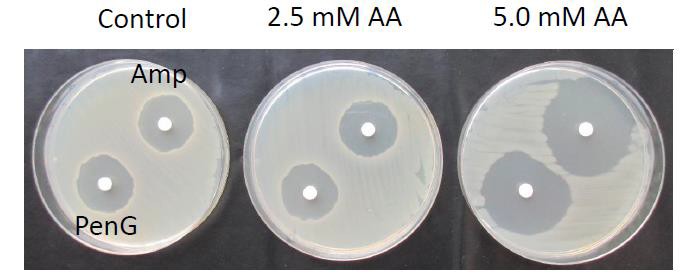
Fig. 1. Potentiating sensitivity of B. subtilis to antibiotics
Mouse models available for Polycystic Kidney Disease (PKD) drug testing drug screening, PKD, kidney fibrosis, pharmacology
drug screening, PKD, kidney fibrosis, pharmacology
Mouse models available for Polycystic Kidney Disease (PKD) drug testing
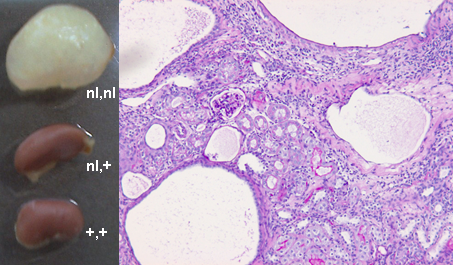
Scientists at Leiden University Medical Center have developed mouse models for Polycystic Kidney Disease (PKD) drug testing.
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is a common genetic disease characterized by progressive development of fluid-filled cysts in both kidneys. The formation of numerous cysts together with interstitial fibrosis usually causes chronic renal failure in 50% of patients by the age of 60 years. ADPKD is a systemic disorder that is caused by mutations in the PKD1 or PKD2 genes. The majority of patients, 85%, carry a mutation in the PKD1 gene.
The first mouse model is a tamoxifen-inducible, kidney epithelium-specific Pkd1-deletion model, which shows that inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys. By selectively inactivating the Pkd1 gene, the embryonic or postnatal lethality is overcome, which makes this model very useful in studying the onset of the disease. A second mouse model has been developed with reduced Pkd1-gene expression. This model is extremely interesting for mimicking the progression and end stages of the disease. Overall, the combination of both mouse models, not hampered by early death, are highly suitable for studying the progression of the disease in vivo and for testing the effect of therapeutic interventions in the different PKD disease stages.
We are interested in speaking with companies with an interest in kidney diseases with a view to a collaboration in this field.
High throughput 3D cell culture assay 3D cell culture, cell spheroids, high-throughput, drug screening, breast cancer, cancer
3D cell culture, cell spheroids, high-throughput, drug screening, breast cancer, cancer
High throughput 3D cell culture assay

Scientist at Leiden University have developed a fast and robust 3-dimensional (3D) cell culture assay.
In this 3D assay, cell speroids are formed within minutes at precise pre-determined positions.This novel 3D cell culture assay is highly suited for high throughput drug screening, especially in cancer research.
The need for better in vitro screening technology is well known and documented in the field. The currently used 2D cell assays are fast and easy to perform, but lack predictive value. Among the various 3D culture platforms, 3D ECM-embedded cell spheroids most closely represent the in vivo tumor microenvironments. However, several technical hurdles preclude the use of such cultures in high content screening (HCS): i) spheroid creation is difficult and poorly reproducible: ii) it is restricted to certain cell types: and iii) spheroid location cannot be carefully controlled, which hampers automated imaging.
Scientists at Leiden University have developed an automated method to create 3D ECM-embedded cell spheroids that overcomes these limitations. Spheroid formation time is strongly reduced compared to other methods (minutes rather than days) and it can be applied to a broad range of cell types including cells which naturally do not form cell-cell contacts, endothelial cells, various cancer cell lines, and primary tumor cell suspensions. For High Content Screening, we are able to produce 1 spheroid per well in 384 well plates or up to 7 patterned spheroids per well in 96 well plates. Importantly, the spheroids have defined x-y-z spatial coordinates allowing automated confocal imaging and image analysis algorithms. A successful proof-of-principle chemical screen was done on breast cancer spheroids identifying compounds affecting cancer cell invasion/migration.

ApoE3Leiden Mouse obesity, diabetes, Artherosclerosis, brown fat, metabolic disease
obesity, diabetes, Artherosclerosis, brown fat, metabolic disease
ApoE3Leiden Mouse
Apolipoprotein E is a constituent of VLDLs, chylomicrons, and HDLs and is essential for receptor-mediated uptake of remnant lipoproteins.
ApoE deficiency in mice leads to elevated plasma cholesterol levels that are due to the accumulation of remnant lipoproteins, and ApoE deficiency is associated with the development of atherosclerosis. In addition, these mice develop a fatty liver when fed normal chow and show a decreased VLDL-triglyceride secretion. In humans, the mutant ApoE3Leiden isoform is associated with a dominantly inherited form of familial dysbetalipoproteinemia.
The ApoE3Leiden gene contains a tandem repeat of codons 120 to 126, yielding a protein of 306 amino acids. Transgenic mice expressing ApoE3Leiden develop hyperlipidemia as a result of defective binding of E3Leiden-containing remnant lipoproteins to the LDL receptor and to the LDL receptor–related protein and are susceptible to diet-induced atherosclerosis.
Apolipoprotein E (ApoE)-deficient mice develop hepatic steatosis and show impaired very low density lipoprotein (VLDL)-triglyceride (TG) secretion. These effects are normalized with the introduction of the human ApoE3 gene. The APOE3Leiden mouse displays a human-like plasma lipid profile and is sensitive to diet-induced hyperlipidemia, obesity and insulin resistance as well as premature atherosclerosis.
Researchers at Leiden University Medical Center (LUMC) are interested in research collaborations using the ApoE3Leiden Mouse including crossbreeding to develop novel mouse models.
New mouse model, expressing human Fc receptors, to test the efficacy of therapeutic antibodies drug delivery, dermatology, cosmetics, antibodies, collaboration
drug delivery, dermatology, cosmetics, antibodies, collaboration
New mouse model, expressing human Fc receptors, to test the efficacy of therapeutic antibodies
Over the years, a lot of therapeutic antibodies have been developed and marketed. These antibodies have the capacity to treat diseases such as rheumatoid arthritis, multiple sclerosis, psoriasis and several forms of cancer.
The potential of antibodies for healthcare is enormous and several new antibodies directed to new targets are under development. It appears very difficult to test the efficacy of the therapeutic antibodies in the first phases of clinical research. This is currently performed in vitro and in mouse models. However, in vitro tests do not represent the broad context of the body, whereas in the mouse the Fc receptors (FcR), which are the main molecules interacting with antibodies, are not identical to human FcR. For this reason there is a strong need for more reliable in vivo test models based on the characteristics of the human body.
To fulfill this need, scientists at Leiden University Medical Center have developed two new mouse models, which, when crossed, will generate a mouse model with human FcR in the absence of mouse FcR. (‘Humanized mouse’). The scientists are looking for a commercial partner to develop the desired mouse model to be able to study the effects of therapeutic antibodies in the human body.
Furthermore, since the copy number and expression of the different members of the FcR family varies between individuals, additional models could be developed, displaying specific types of human FcR, which will then be representative for a specific part of the population. The availability of this kind of models will allow for the development of personalized therapeutic strategies, dependent on the patient’s FcR expression. We are looking for a biotechnology/pharmaceutical partner with experience in drug testing and/or the development of therapeutic antibodies.
Fig. 1 The in vivo test tube for the validation of human therapeutic monoclonal antibodies and the analysis of human pathogenic antibodies
Therapeutic intervention in Facioscapulohumeral muscular dystrophy (FSHD) muscular dystrophy, FSHD, research, gene therapy, epigenetic therapy, exon skipping, small molecule therapy
muscular dystrophy, FSHD, research, gene therapy, epigenetic therapy, exon skipping, small molecule therapy
Therapeutic intervention in Facioscapulohumeral muscular dystrophy (FSHD)

Scientists at Leiden University Medical Center (LUMC), in collaboration with other academic institutions, have discovered and developed novel target mechanisms, as well as in-vitro and in-vivo models, for the development of therapeutic interventions in Facioscapulohumeral Muscular Dystrophy (FSHD).
FSHD is the third most common muscular dystrophy in man with an estimated incidence of 54 per million. Patients suffer from progressive and irreversible weakness and wasting of the facial, shoulder and upper arm muscles. Approximately 20% of gene carriers become wheelchair dependent. There is no cure for FSHD.
Scientists at LUMC, in collaboration with other academic institutions, have discovered two novel target mechanisms whereby the two forms of FSHD can arise. The mechanisms represent targets for therapeutic intervention.
In addition, cell lines and mouse models of FSHD have been developed and can be used to further research the disease and/or to screen and validate potential therapeutics.
The collaborating institutions represent world-leading expertise in the field of FSHD and can also provide ongoing expertise.
Partner companies are now sought for research collaborations in this field, and licensing of key technologies available at the institutions.
Increasing the efficacy of Vancomycin - Natural compounds to reduce resistance antibiotics, antibiotic resistance, antibiotic adjuvant, vancomycin
antibiotics, antibiotic resistance, antibiotic adjuvant, vancomycin
Increasing the efficacy of Vancomycin - Natural compounds to reduce resistance

Scientists at Leiden University's Institute of Biology have found a compound that decreases the resistance of vancomycin-resistant bacteria.
The sensitizing effect of this compound increases further when the activity of other vancomycin-resistance enzymes are targeted. Their work offers new perspectives for the treatment of diseases associated with vancomycin-resistant pathogens and for the development of drugs that target vancomycin resistance.
Currently, the scientists are seeking partnerships for the next stage of development.
Vancomycin is nowadays primarily used to treat serious infections caused by gram-positive bacteria which are known or suspected to be resistant to other antibiotics. Acquired resistance to vancomycin by gram-positive bacteria, mediated via a plasmid-transmittable vancomycin resistance cluster, is a growing problem in the nosocomial environment. Vancomycin resistance evolved in common pathogenic organisms during the 1990s and 2000s, including vancomycin-resistant Staphylococcus aureus .
Vancomycin is regarded as a last resort drug against infections with Gram-positive pathogens, witnessed by its’ listing on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system.
Vancomycin targets the cell wall by specifically binding to the termini of the peptidoglycan precursor lipid II, prior to its incorporation into mature. The resulting weakening of the cell-wall integrity then leads to osmolysis. The termini are universally conserved in bacteria. The Leiden scientists have found that the addition of a natural compound to the vancomycin-resistant model organism Streptomyces coelicolor, as well as clinical isolates of Enterococcus faecium dramatically enhanced the efficacy of vancomycin. Fluorescence imaging of S. coelicolor mycelia using BODIPY-vancomycin showed that the enhanced vancomycin sensitivity correlated directly to increased binding of the antibiotic to the cell wall.
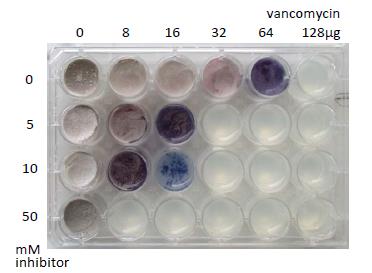
Fig. 1. Reduction of vancomycin tolerance of S. coelicolor M145 (a naturally vancomycin-resistant model organism)
New approach for oligonucleotide-mediated exon skipping in neurodegenerative diseases neurodegenerative diseases, exon skipping, SCA3,SCA1, DRPLA
neurodegenerative diseases, exon skipping, SCA3,SCA1, DRPLA
New approach for oligonucleotide-mediated exon skipping in neurodegenerative diseases
Scientists at the Leiden University Medical Center have developed a new approach for antisense oligonucleotide-mediated exon skipping in neurodegenerative diseases. This method allows for the removal of exons containing either the proteolytic cleavage site or the mutation in the coding region of the gene that is causative for the neurodegeneration.
There are several neurodegenerative diseases that are caused by cleavage of a protein by proteolytic cleavage, resulting in a toxic protein fragment that causes neurodegeneration. Other neurodegenerative diseases are caused by a mutation in the coding region of a gene, resulting in a mutated protein that has gained a toxic function as a result of this mutation. Currently there is no cure on the market for these diseases. Current treatments are symptomatic.
Partner companies are now sought for research collaborations in this field, and licensing of key technologies available at the institutions. Specifically we are looking for companies with a franchise in the treatment of neurodegenerative disorders.
Metal and Alloy Nanoparticle Production through Cathodic Corrosion alloy, antibacterial, catalysis, cathodic corrosion, colloidal, electrochemical methods, high purity, metal, metal alloy, metal nanoparticle, nanocomposite, nanoparticle, nanoparticle production, silver nanoparticle
alloy, antibacterial, catalysis, cathodic corrosion, colloidal, electrochemical methods, high purity, metal, metal alloy, metal nanoparticle, nanocomposite, nanoparticle, nanoparticle production, silver nanoparticle
Metal and Alloy Nanoparticle Production through Cathodic Corrosion
Background
Leiden University has developed a radically different method for large scale production of diverse metal nanoparticles and their alloys. The size of nanoparticles range from 2 – 100 nm in liquids.
A Proof of Principle has been demonstrated and a larger production unit became operational recently. The new unit will make it possible to provide research institutes and industry samples for further evaluation.
Technology Overview
Cathodic corrosion for producing nanoparticles was (re)discovered when trying to control the electrochemical etching of a scanning tunneling microscopy (STM) tip. We have shown that the metal nanoparticles (NPs) and their alloys can be easily produced by using cathodic corrosion and their sizes and compositions can be controlled. The produced NPs were shown to have high catalytic activity and superior to the commercial ones. Since cathodic corrosion is radically different from all other existing methods of NP synthesis, its ability for tuning properties of NPs is still relatively unexplored, and hence improved characteristics are still expected. Given the enormous simplicity and versatility of the method, we believe that cathodic corrosion has unique potential.
This method includes also the inhibition of agglomeration, the scaling up of NP production and the in-situ impregnation of functional nanocomposites.
More information is to be found in the following scientific publications:
https://pubs.acs.org/doi/abs/10.1021/acsami.7b18105
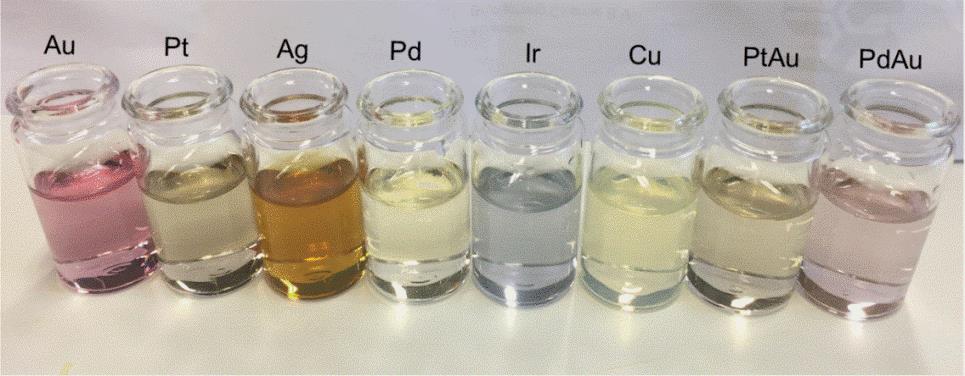
 Figure 1. Photographs of the colloidal nanoparticles (NPs) generated by the cathodic corrosion method. (a) monometallic Au, Pt, Ag, Pd, Ir, Cu, and binary PtAu and PdAu alloy nanoparticles (NPs); (b) AgxAu100-x (atomic ratio) nanoalloys with x = 10, 30, 50, 70, and 90.
Figure 1. Photographs of the colloidal nanoparticles (NPs) generated by the cathodic corrosion method. (a) monometallic Au, Pt, Ag, Pd, Ir, Cu, and binary PtAu and PdAu alloy nanoparticles (NPs); (b) AgxAu100-x (atomic ratio) nanoalloys with x = 10, 30, 50, 70, and 90.
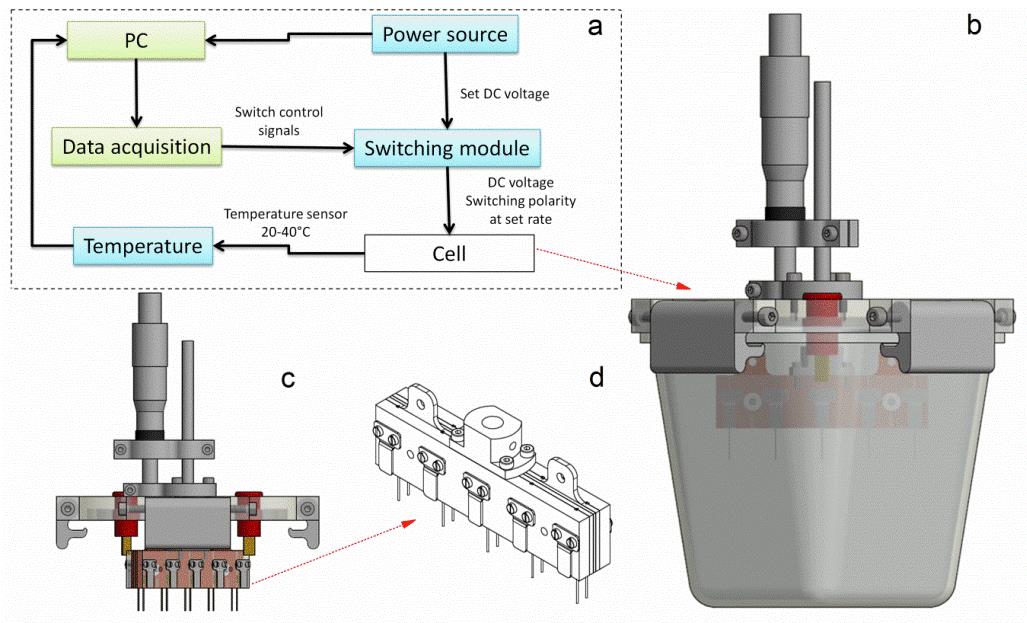
Figure 2. Schematic illustration of the ‘comb-electrode’ setup for the cathodic corrosion method . The block diagram of the global design of the setup (a), the complete cell system (b), the electrode feeding component (c) and an enlarged ‘comb’ consisting of 5 electrode pairs (d). A micrometer screw mounted on the comb electrodes was used to precisely adjust their submersion depth (measured from the moment the electrode touches the liquid surface) in the liquid.
Adjuvant Compounds for Conjugated Synthetic Vaccines adjuvant, agonist, cell, activation, immune response, immunological ligands, synthetic vaccine
adjuvant, agonist, cell, activation, immune response, immunological ligands, synthetic vaccine
Adjuvant Compounds for Conjugated Synthetic Vaccines
Background
Synthetic vaccines consist of well-defined chemically produced molecules and require two components: an antigen and an immune-stimulating adjuvant. In peptide-based synthetic vaccines the antigen is a peptide that contains a sequence (the epitope) that is specifically recognized by the T cell immune system while the adjuvant is often a ligand of one of Toll-like receptors (TLR’s) that provides a “danger signal” and expands specific T cells. In this invention an improved adjuvant is described that can be used as the entity covalently attached to any synthetic peptide sequence.
Tech Overview
This technology builds on a well-known design of conjugated vaccines comprising synthetic lipopeptides, in which the lipophilic TLR-2 agonist is attached to the N-terminus of synthetic antigenic peptide. Researchers from Leiden University have previously shown that classical TLR2 ligand-conjugated peptide vaccines has strong T cell activation potency in vivo and can control aggressive tumor growth. The compound contains a defined lipidcomponent which can be covalently attached to any synthetic peptide sequence. The adjuvant compound retains its strong adjuvanticity upon conjugation but possesses much improved physicochemical properties as compared to established lipid-based synthetic vaccines. The synthesis of the conjugatable TLR-2 agonist is accomplished by the methods of solution phase organic chemistry and subsequently it is readily coupled to a peptide sequence preassembled on solid-phase by standard method of peptide chemistry. The finished conjugate is liberated from the solid-phase and purified by the techniques common in the preparation of peptide-based vaccines.
The conjugable TLR2 lipopeptide adjuvant platform is suited to augment the immunogenicity of synthetic peptidebased vaccines to any pathogen or malignancy of which sequence information of immunogenic epitopes is
available. Especially for personalized vaccines for cancer patients this adjuvant offers a flexible system to swiftly conjugate multiple peptide sequences of tumor-specific mutated neo-epitopes.
Reconstructed Human Skin Models for Research and Screening Purposes dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
Reconstructed Human Skin Models for Research and Screening Purposes
Background:
Functional 3D reconstructed human skin equivalents (HSEs) can be used for drug testing that avoids the excessive use of experimental animals. HSEs are three-dimensional systems that recapitulate most of the in vivo characteristics and in which cellular processes may be normalized compared to conventional monolayer cultures. These in vitro 3D-HSEs are the result of more than 25 years of research and development by scientists from Leiden University Medical Centre (LUMC) (The Netherlands).
Within the Department of Dermatology, LUMC provide both healthy and diseased in vitro HSEs for animal‐free compound screening and safety testing services and for co‐development activities. HSEs are used in new product development and substantiation of product claims in cosmetic, pharmaceutical, food and environmental industries, but also in fundamental research within the field of experimental dermatology. These HSEs enable genomic, proteomic and drug development research not possible with traditional, unrepresentative or even unavailable animal models, monolayer cell cultures or clinical test procedures. Uniquely, all in vitro HSEs are fully customizable to meet both the scientific and commercial demands of the customer.
Technology Overview:
Currently LUMC offer four different HSEs; the Leiden Epidermal model (LEM), the Full-Thickness model (FTM), the Fibroblasts-Derived Matrix model (FDM) and the Ex-vivo human skin (ExHs).

(Figure 1.)
- LEM consists of keratinocytes seeded on a non-cellular matrix (e.g. inert filter membrane or de-epidermized dermis. These epidermal models are suitable for e.g. skin toxicity, irritation, or penetration tests. In 2008, the researchers pre-validated the Leiden epidermal model (LEM) for skin irritation and corrosion (El Ghalbzouri et al., 2008).
- FTM consists of keratinocytes, melanocytes and a fibroblast-populated three-dimensional collagen matrix. This model closely resembles native human skin. This full-thickness skin model can be used for tests, predictive screening and research on for example wound healing that requires the complexity of human skin, i.e. where the interaction between epidermal and dermal cells is crucial.
- FDM is similar to the FTM model, but the dermal compartment consists of human fibroblast-derived extracellular matrix. This model can be used as a tool to evaluate the effect of e.g. ingredients on dermal processes in skin aging.
- By using intact fresh skin biopsies, the researchers can perform short-term studies on human skin. By placing these skin samples onto an inert filter they can culture these ex vivo skin models (ExHs) up to 1 week.
All models can be used for predictive screening or contract research that requires the complexity of human skin. Variations of these models can be generated by incorporation of other cell types (e.g. melanocytes, different types of fibroblasts, tumour cells etc.). The different models can also be grown at different oxygen concentrations.
Within the Department of Dermatology, LUMC have exploited the HSEs for more than ten years as a tool to conduct contract research for a number or large and medium size enterprises. These projects are focused on different aspects of healthy and diseased skin, such as; skin biology, dermal interactions, skin aging, wound healing, scars, treatment of bacterial infections by antibiotics and/or anti-microbial peptides. Some examples are given in Figure 2.
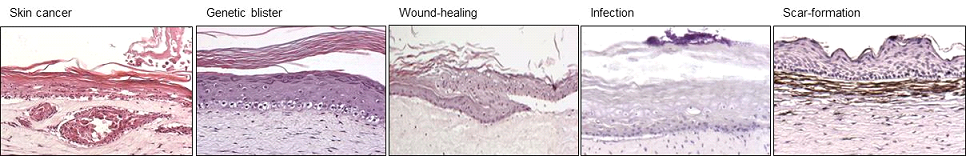
(Figure 2.)
Imaging tools to characterise migration and interaction of parasites within their host parasite, malaria, vaccine, molecular imaging, formulation, parasites, fluorescence, schistosoma, hookworm, bacteria
parasite, malaria, vaccine, molecular imaging, formulation, parasites, fluorescence, schistosoma, hookworm, bacteria
Imaging tools to characterise migration and interaction of parasites within their host

Background:
With roughly half of the world’s population at risk, the treatment and prevention of parasitic infections remains a medical priority. Parasites typically display migratory behavior, disregard anatomical barriers and interact with their host at several levels during their life cycle. Very little is known about the biology that drives the migration and interaction of parasites inside their intermediate or definite hosts. A better understanding of this is essential to help improve the design and development of vaccines.
Using malaria parasites as an example, LUMC researchers reasoned that imaging-based tracking of individual parasites of different native malaria species can help understand the interaction of these parasites with their host. LUMC researchers have developed imaging tools to assess the motility of live attenuated parasite vaccine formulations in human tissue.
Technology Overview:
LUMC researchers have developed molecular imaging technology that uses a fluorescent/radioactive tracer to label parasites. Malaria parasites can be labelled either in the mosquito host or under in situ conditions. Next to the this labelling, LUMC has developed a complementary software package that allows for quantitative analysis of parasite behaviour in tissue.
This molecular imaging technology allows for quantification of the behavioural differences between wild type parasites and radiation-, chemically-, or genetically- attenuated vaccine derivatives. Feedback from imaging these parasites provides a reference to which vaccine administration routes and future vaccine formulations can be benchmarked. The imaging technology also supports the isolation of distinct behavioural features that could be critical in interaction with the immune system.
Because the technology is based on chemistry rather than GMO technologies, it allows for the first time imaging of wild-type parasites including those species for which GMOs do not exist. In addition, the molecular imaging technology has been successfully applied to Schistosoma mansoni and Necator americanus parasites.
In addition to an established application in the field of parasites, the developed technology could also be applied to monitor other pathogens e.g. bacterial infections or colonisation models .
Benefits:
These technologies provide a means to image and quantify the infectious behaviour of parasites and attenuated whole-body parasite vaccines. The technologies:
- Allow for straight forward fluorescence imaging of parasites
- Allow for quantification of parasite migration in human tissue
- Are suitable for clinical translation.
Opportunity:
We are looking for partner(s) to license the technology for commercialization to and/or to fund research into refinement and clinical translation of the technology.
Platinum metal-based anticancer and antibacterial drugs metallodrugs, bacteria, cancer, pharmaceutical industry, biotech/medical companies
metallodrugs, bacteria, cancer, pharmaceutical industry, biotech/medical companies
Platinum metal-based anticancer and antibacterial drugs
Technology Overview:
Researchers at Leiden University have developed novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents. A family of related ligands can be used in the formulation of the compounds, to tune solubility and activity. The compounds are very stable due to the tetradentate nature of the ligands, coupled with a tetracoordinated (d8) metal center.
While these novel compounds are both simple and cheap to synthesize, they could provide an interesting new alternative for platinum-based anticancer drugs (platinum based drugs such as cisplatin, oxaliplatin and carboplatin already have FDA-approval and are used widely). These new compounds are all the more interesting, because micromolar to nanomolar activities have been observed on cancer cell lines that are resistant to cisplatin.
However the activities are not limited to cancer therapy; applications as an antibacterial agent are also possible. The exact activities of the different compounds depend on the entire compound, while the mode of action depends critically on the nature of the metal.
Potential applications:
- Cancer therapy
- Antibacterial agent
State of development:
The compounds have been synthesized and characterized. In vitro and in vivo (mouse model) experiments have been conducted that have confirmed the high potential of the compounds; in vitro experiments have revealed that the cytotoxicity of some derivatives on human cancer cell lines are among the highest ever reported in the literature, while preliminary in vivo experiments in a mouse model show a decrease in tumor size.
Opportunity:
The researchers are looking for partners to take this invention to the next stage, which would be doing experiments to confirm the biological target, mode of action, and refine efficacy in vivo.
Novel approach to conjugate adjuvants to live-attenuated vaccines parasite, malaria, vaccine, molecular imaging, formulation, parasites, fluorescence, schistosoma, hookworm, bacteria
parasite, malaria, vaccine, molecular imaging, formulation, parasites, fluorescence, schistosoma, hookworm, bacteria
Novel approach to conjugate adjuvants to live-attenuated vaccines
Background:
Live attenuated or inactivated organisms are the most efficacious and cost-effective vaccines available. In order to decrease production costs and increase immunogenicity of these vaccines, they can be administered together with adjuvanting molecules. Adjuvants increase the potency of the vaccine. However in live attenuated vaccines, where the vaccine organism may migrate or distribute in the host, co-delivery of vaccine and adjuvant to the same immune cell is challenging.
Particularly for organisms which are naturally very low immunogenic, such as malaria parasites, the conjugation of adjuvanting molecules to the outside of the organism would be an ideal solution.
Technology Overview:
LUMC researchers have developed a novel adjuvant technology, that amplifies the interaction of the live-attenuated (sporozoite) vaccine with the host immune system. This technology utilises supramolecular chemistry to pre-target the organism and secondly to conjugate the adjuvant and enhance the immune response. Uniquely, this technology can be applied to both wild-type and attenuated sporozoites and does not require genetic modification of the organism. It can also be used to induce or polarize an immune response at any point during the development of the pre-erythrocytic malaria parasites and/or at specific anatomical locations.
Research so far has focused on malaria. However, the developed technology is non-specific and has the potential to be used for other single-cell pathogens such as bacteria or multicellular organisms such as helminths.
Benefits:
These technology provides a means to enhance the immune response towards live-attenuated parasite or bacterial vaccines. The technology:
- Is a novel approach to conjugate adjuvants to live-attenuated vaccines
- Preserves the viability of the live vaccine organism
- Does not require genetic modification
- Allows for enhancement of the immune response
- Is suitable for clinical translation.
Opportunity:
We are looking for partner(s) for joint research, development and clinical translation of this technology.
Photo activated cancer prodrug metallodrugs, light activation, drug delivery, prodrugs, pharmaceutical industry, biotech/medical companies
metallodrugs, light activation, drug delivery, prodrugs, pharmaceutical industry, biotech/medical companies
Photo activated cancer prodrug
Technology Overview:
Researchers at Leiden University in collaboration with researchers from Texas State University have developed a novel photo activated anticancer prodrug. This prodrug consists in a rigidin analogue caged by a ruthenium polypyridyl complex that can be released upon green light irradiation. The photocaged rigidin inhibitor is a microtubule polymerization inhibitor, and as such disrupts the formation of tumor vasculature.
Microtubule-targeting agents have been used in clinic for over 50 years, but they can be very toxic for the patient. The successful caging and release upon irradiation of the inhibitor means that it is now possible to keep the systemic concentration of the (pro)drug at a higher level than the level of toxicity, without such a high concentration causing significant biological effects. By shining light at the tumour site, a high dose of the inhibitor is only released locally (or in other words, the caged inhibitor is “hidden” in the dark).
Because the agent activation is irreversible, activity will be retained after light irradiation has been removed. Also, unlike in photodynamic therapy, where oxygen concentration in the irradiated tumor should be high enough, these new compounds remain active under hypoxic conditions (1% oxygen) as their activation mechanism does not rely on the presence of dioxygen.
Finally, attachment of the ruthenium-based caging significantly increases the water solubility of the drug in the dark.
Potential applications:
- Cancer therapy
State of development:
The 7-deazahypoxanthine synthetic analogues of marine alkaloid rigidins have shown promising anticancer activities; they were found to be highly effective in eradicating cancer cells in cell cultures at low doses (double- to single-digit nanomolar antiproliferative IC50 values) and showed statistically significant tumor size reduction in a colon cancer mouse model at nontoxic concentrations.
Opportunity:
The researchers are looking for partners from industry to take this invention to the next stage, which would be to carry out extensive in vivo (mouse model) studies.
IP Status:
The rigidin analogues have been patented by New Mexico Tech and Texas State University jointly, while a patent application has been filed for the caging technology by Texas State University and Leiden University jointly.
Reconstructed Human Skin Models for Research and Screening Purposes Four human skin equivalents that can be used for predictive screening or contract research that requires the complexity of human skin.
Four human skin equivalents that can be used for predictive screening or contract research that requires the complexity of human skin.
Reconstructed Human Skin Models for Research and Screening Purposes
Background
Functional 3D reconstructed human skin equivalents (HSEs) can be used for drug testing that avoids the excessive use of experimental animals. HSEs are three-dimensional systems that recapitulate most of the in vivo characteristics and in which cellular processes may be normalized compared to conventional monolayer cultures. These in vitro 3D HSEs are the result of more than 25 years of research and development by scientists from Leiden University Medical Center (LUMC) (The Netherlands). Within the Department of Dermatology, LUMC provide both healthy and diseased in vitro HSEs for animal-free compound screening and safety testing services and for co-development activities. HSEs are used in new product development and substantiation or product claims in cosmetic, pharmaceutical, food and environmental industries, but also in fundamental research within the field of experimental dermatology. These HSEs enable genomic, proteomic and drug development research not possible with traditional, unrepresentative or equally unavailable animal models, monolayer cell cultures or clinical test procedures. Uniquely, all in vitro HSEs are fully customizable to meet both the scientific and commercial demands of the customer.
Technology Overview
Currently LUMC offer four different HSEs; the Leiden Epidermal model (LEM), the Full-Thickness model (FTM), the Fibroblasts-Derived Matrix model (FDM) and the Ex-vivo human skin (ExHs) (Figure 1).
1. LEM consists of keratinocytes seeded on a non-cellular matrix (e.g. inert filter membrane or de-epidermized dermis. These epidermal models are suitable for e.g. skin toxicity, irritation, or penetration tests. In 2008, the researchers pre-validated the Leiden epidermal model (LEM) for skin irritation and corrosion (El Ghalbzouri et al., 2008).
2 FTM consists of keratinocytes, melanocytes and a fibroblast-populated three-dimensional collagen matrix. This model closely resembles native human skin. This full-thickness skin model can be used for tests, predictive screening and research on for example wound healing that requires the complexity of human skin, i.e. where the interaction between epidermal and dermal cells is crucial.
3. FDM is similar to the FTM model, but the dermal compartment consists of human fibroblast-derived extracellular matrix. This model can be used as a tool to evaluate the effect of e.g. ingredients on dermal processes in skin aging.
4. By using intact fresh skin biopsies, the researchers can perform short-term studies on human skin. By placing these skin samples onto an inert filter they can culture these ex vivo skin models (ExHs) up to 1 week.

(Figure 1.)
All models can be used for predictive screening or contract research that requires the complexity of human skin. Variations of these models can be generated by incorporation of other cell types (e.g. melanocytes, different types of fibroblasts, tumour cells etc.). The different models can also be grown at different oxygen concentrations.
Within the Department of Dermatology, LUMC have exploited the HSEs for more than ten years as a tool to conduct contract research for a number or large and medium size enterprises. These projects are focused on different aspects of healthy and diseased skin, such as; skin biology, dermal interactions, skin aging, wound healing, scars, treatment of bacterial infections by antibiotics and/or anti-microbial peptides. Some examples are given (Figure 2).

(Figure 2.)
Applications
There is an unmet clinical need for dermatology to develop therapies for a large number of skin diseases, such as skin cancer (eg melanoma), wound healing, bacterial wound infections, and eczema. There are currently no suitable in vitro models to screen and validate novel targets, and test the effects of potential new therapies in vitro . Cosmetics, Food, Chemical, Pharmaceutical, Medical devices.
Opportunity
The models are available for contract research and for co-development activities.
Keywords: dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
Novel Pharmacologial Chaperones for Improved Enzyme Replacement Therapy A small molecule with potential to be used as part of a new treatment for Fabry patients in combination with enzyme replacement therapy.
A small molecule with potential to be used as part of a new treatment for Fabry patients in combination with enzyme replacement therapy.
Novel Pharmacologial Chaperones for Improved Enzyme Replacement Therapy
Background
Enzyme replacement therapy (ERT) is a very costly therapeutic treatment. The costs of ERT starting in the symptomatic stage are between €9 - €10 million (£ 7.9 - £ 8.8 million, $13.0- $14.5 million) during a patient's lifetime. The combination of small pharmacological chaperones (PCs) with ERT has shown a synergic effect in improved enzyme activity and reduction of toxic metabolites. Therefore, it is expected that the co-treatment PC-ERT may reduce the amount of ERT necessary to achieve the desired pharmacological effect and therefore may lower lysosomal storage diseases (LSDs) treatment costs.
Technology Overview
Leiden University researchers have developed a new class of pharmacological chaperones (PCs) named cyclosulfamidates. α-galactose configured cyclosulfamidate reacts reversibly with α-gal A and stabilized the enzyme in vitro and in situ. They have shown that α-cyclosulfamidate is able to stabilize the recombinant human enzyme in vitro and in situ cell experiments and increased α-gal A activity is observed in the medium of the cells co-treated with PC and recombinant α-gal A. This increase is translated in a slightly higher activity in the cells treated with ERT and PC combination for 24 h and 4 days.
The researchers also quantified the amount of Gb3 and lysoGb3 in treated and untreated cultured fibroblast lysates and they interestingly observed that the combination of ERT and α-cyclosulfamidate has a similar effect that ERT effect when compared to ERT alone. In conclusion, herein they describe the potential of a new small molecule, α-cyclosulfamidate, as stabilizer of α-gal A and its potential as new treatment for Fabry patients in combination with ERT.
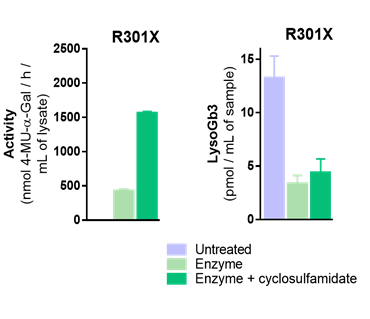 A structure activity relationship (SAR) on the galactose configured cyclosulfamidate is ongoing. In addition, glucose configured cyclosulfamidates as new potential pharmacological chaperones for Pomple and Gaucher Disease are comparatively studied.
A structure activity relationship (SAR) on the galactose configured cyclosulfamidate is ongoing. In addition, glucose configured cyclosulfamidates as new potential pharmacological chaperones for Pomple and Gaucher Disease are comparatively studied.
Applications
This invention provides various combinations of enzyme replacement therapy or (multi)gene therapies with a pharmacological chaperone for the treatment of lysosomal storage diseases and glycosidase deficiency related diseases, particularly Fabry, Gaucher or Pompe disease. This combination optimizes the clinical benefit, i.e., reduced enzyme treatment, while minimizing disadvantages associated with ERT.
Proteomining: An Integrated Approach to Mine for Novel Bioactive Compounds Produced by Actinomycetes Exploits the application of genome sequencing with metabolite identification methods, activity assays and proteomics or RNAsequencing.
Exploits the application of genome sequencing with metabolite identification methods, activity assays and proteomics or RNAsequencing.
Proteomining: An Integrated Approach to Mine for Novel Bioactive Compounds Produced by Actinomycetes
Background
The expression of natural products produced by bacteria fluctuate with growth conditions, with a close correlation between the compounds and the gene expression that encode their biosynthesis. Some of the most promising compounds are only expressed when cultivated under specific conditions, and therefore have been missed in the traditional screening campaigns by BigPharma. These are also referred to as 'cryptic' natural products, often in association with antibiotic activity. An important step in the drug discovery program is to identify the genes that are responsible for the production of the bioactive compound of interest. A method that allows the rapid linkage of biosynthetic machinery (and hence biosynthetic genes) to a compound of interest is crucial to obtain rapid insight into the nature of observed bioactivity. This will speed up the replication and drug discovery.
Technology Overview
Researchers at Leiden University have developed an integrated approach to mine for novel bioactive compounds produced by bacteria (e.g. Actinobacteria, Bacilli, Burkholderia, Pseudomonas), that exploits the application of genome sequencing in combination with metabolite identification methods, activity assays and proteomics or RNAsequencing. This approach allows identification of product, bioactivity and protein, whereas the protein then connects directly to the genome. A unique selling point for the technology is that it allows fast coupling of the genome to the metabolome and vice versa, without requirement of any knowledge on the chemical structure of the bioactivity of interest. That means it can also be used to find the biosynthetic genes for molecules that have never been seen before. In summary, the researchers developed an integrated method, which they named Natural Product Proteomining (in short, Proteomining), that accelerates the identification of genes that are responsible for the production of any bioactivity in microorganisms, which can be measured and whose activity fluctuates with growth conditions. The application lies in two directions, namely (1) finding a biosynthetic gene cluster or enzyme for a given bioactive molecule; or (2) finding a bioactive compound encoded by a given biosynthetic gene cluster.
Details and State of Development:
Application for the identification of known antibiotics and other bioactive compounds (validation) has been demonstrated, from Bacillus and Streptomyces species.
Applications
1. Discovery of microbial natural products with detectable bioactivity
2. Discovery of the biosynthetic gene cluster for a given bioactivity of interest.
3. Specifically, discovery of natural products with application as antibiotic, anticancer agent, antifungal, as anthelmintic or with other detectable feature (could also just be a mass spec peak).
Opportunity
Leiden University welcomes both licensing and R&D partnering inquiries.
Type 1 Diabetes Neo-Epitopes: Autoimmunity Against a Defective Ribosomal Insulin Gene Product A new neo-epitope generated by defective ribosomal products (DRiPs) from the proinsulin gene.
A new neo-epitope generated by defective ribosomal products (DRiPs) from the proinsulin gene.
Type 1 Diabetes Neo-Epitopes: Autoimmunity Against a Defective Ribosomal Insulin Gene Product
Background
Type 1 diabetes (T1D) is an autoimmune disease where the immune system destroys the insulin-producing pancreatic beta cells. These cells are insulin factories dedicated to the maintenance of glucose homeostasis; insulin, stored in secretory granules, represents 10–15% of the protein content of these cells. Studies of samples from humans with T1D and mouse models of the disease indicate that native insulin and its precursors act as primary autoantigens, and fragments of the preproinsulin peptide have been identified as main targets of cytotoxic islet-autoreactive CD8+ T cells in human T1D. In autoimmune disease, there is increasing evidence that local inflammation or other forms of stress combined with genetic disposition leads to the generation and the accumulation of aberrant or modified proteins (neo-epitopes) to which central tolerance is lacking and thereby triggering autoimmunity. Until now, neo-epitopes have been shown to be generated through transcriptional, post-transcriptional and post-translational processes. However, an important class of neo-epitopes could be generated through non-conventional translational events and this technology is the first evidence of a naturally processed and presented epitope derived from nonconventional islet proteins.
Technology Overview
Scientists at Leiden University Medical Center have recently discovered a new neo-epitope generated by defective ribosomal products (DRiPs) from the proinsulin gene. Within the mRNA, the researchers have found an alternative open reading frame encoding a highly immunogenic polypeptide that is targeted by T cells in type 1 diabetes (T1D) patients. They show that cytotoxic T cells directed against the N-terminal peptide of this nonconventional product are present in the circulation of individuals diagnosed with T1D, and provide direct evidence that such CD8+ T cells are capable of killing human beta cells and thereby may be diabetogenic. This is the first evidence of a naturally processed and presented epitope derived from nonconventional islet proteins leading to the destruction of human beta cells by cytotoxic CD8+ T cells. They propose a new pathway of beta cell destruction by the immune system in which the generation of a neoepitope, such as INS-DRiP, plays a central role. Their findings support the emerging concept that beta cells are destroyed in T1D by a mechanism comparable to classical antitumour responses whereby the immune system has been trained to survey for dysfunctional cells in which errors have accumulated. This invention reveals a potential new mechanism underlying the pathology of T1D, and may allow the development of novel T1D diagnostics and therapies (Nat Med. 2017 Apr;23(4):501-507).
Details and State of Development:
Proof of concept.
Applications
- Novel T1D diagnostics
- Novel T1D therapies
Opportunity
- Research collaboration to further unravel the mechanism of INS-DriP formation
- Development of novel T1D diagnostics and therapies
Ubiquitin Variants as Antiviral Agents Structurally diverse, virus-specific deubiquitinating enzymes can be selectively targeted through rational protein design technology.
Structurally diverse, virus-specific deubiquitinating enzymes can be selectively targeted through rational protein design technology.
Ubiquitin Variants as Antiviral Agents
Background
Emerging viruses pose a tremendous challenge to human health. Recent Middle East respiratory syndrome coronavirus (MERS-CoV) virus emergence and a subsequent array of reported human cases, or which 35% are lethal, exemplify the continued threat or (re) emerging viruses to human health, and our inability to rapidly develop effective therapeutic countermeasures. MERS-CoV and the Crimean-Congo hemorrhagic fever virus (another re-emerging lethal virus causing increasing problems) encode deubiquitinating (DUB) enzymes that are critical for viral replication and pathogenicity which makes them highly attractive antiviral drug targets. The fact that these and other viruses bind the cellular protein ubiquitin can now be used as a basis for development or a highly innovative antiviral strategy.
Technology Overview
Besides vaccines, which are arguably the most desirable remedies against virus infections, specific antiviral drugs are of pivotal importance. Especially for re-emerging virus infections such as those causing MERS and CCHF in acute outbreak situations these drugs provide immediate treatment options for infected patients and prophylactic medicine for people at acute risk of infection, such as medical personnel and close relatives. The standard approach is to use generic antivirals (ribavirin) or general immune boosters (interferon) or look for small chemical compounds that target viral or host enzymes. All of these are often ineffective and have major side-effects. This proof-of-principle work now shows that structurally diverse, virus-specific deubiquitinating enzymes can be selectively targeted with very high affinity binders based on the natural ligand ubiquitin itself, which opens new avenues for quickly developed molecularly tailored therapy across a broad spectrum of viral pathogens that infect humans, livestock and plants. PLoS Pathog 13 (5): e1006372).
Adjuvant Compounds for Conjugated Synthetic Vaccines Compounds which retain their strong adjuvanticity upon conjugation but possess much improved physicochemical properties
Compounds which retain their strong adjuvanticity upon conjugation but possess much improved physicochemical properties
Adjuvant Compounds for Conjugated Synthetic Vaccines
Background
Synthetic vaccines consist of well-defined chemically produced molecules and require two components: an antigen and an immune-stimulating adjuvant. In peptide-based synthetic vaccines the antigen is a peptide that contains a sequence (the epitope) that is specifically recognized by the T cell immune system while the adjuvant is often a ligand of one of Toll-like receptors (TLR’s) that provides a “danger signal” and expands specific T cells. In this invention an improved adjuvant is described that can be used as the entity covalently attached to any synthetic peptide sequence.
Technology Overview
This technology builds on a well-known design of conjugated vaccines comprising synthetic lipopeptides, in which the lipophilic TLR-2 agonist is attached to the N-terminus of synthetic antigenic peptide. Researchers from Leiden University have previously shown that classical TLR2 ligand-conjugated peptide vaccines has strong T cell activation potency in vivo and can control aggressive tumor growth. The compound contains a defined lipid-component which can be covalently attached to any synthetic peptide sequence. The adjuvant compound retains its strong adjuvanticity upon conjugation but possesses much improved physicochemical properties as compared to established lipid-based synthetic vaccines. The synthesis of the conjugatable TLR-2 agonist is accomplished by the methods of solution phase organic chemistry and subsequently it is readily coupled to a peptide sequence pre-assembled on solid-phase by standard method of peptide chemistry. The finished conjugate is liberated from the solid-phase and purified by the techniques common in the preparation of peptide-based vaccines. The conjugable TLR2 lipopeptide adjuvant platform is suited to augment the immunogenicity of synthetic peptide-based vaccines to any pathogen or malignancy of which sequence information of immunogenic epitopes is available. Especially for personalized vaccines for cancer patients this adjuvant offers a flexible system to swiftly conjugate multiple peptide sequences of tumor-specific mutated neo-epitopes.
Details and State of Development:
A solution synthesis of a conjugatable derivative of the TLR-2 agonist on gram-scale is developed as well as a general approach to the solid-phase synthesis of the lipopeptides connected to this adjuvant. Immunological characterization of the TLR-2 agonist has shown:
- Strong TLR2 activation of reporter cell lines;
- Functional maturation of human dendritic cell cultures; and
- Improvement of antigen presentation of conjugated synthetic peptide harboring neo-epitope sequence to melanoma patient-derived human T cells
Applications
1. Peptide based vaccines
2. Personalized vaccines
Keywords: adjuvant, agonist, cell, activation, immune response, immunological ligands, synthetic vaccine
Metal and Alloy Nanoparticle Production through Cathodic Corrosion A radically different method for large scale production of diverse metal nanoparticles and their alloys
A radically different method for large scale production of diverse metal nanoparticles and their alloys
Metal and Alloy Nanoparticle Production through Cathodic Corrosion
Background
Leiden University has developed a radically different method for large scale production of various metal nanoparticles and their alloys. The size of nanoparticles range from 2 - 100 nm in liquids.
A Proof of Principle has been demonstrated and a larger production unit became operational recently. The new unit will make it possible to provide research institutes and industry samples for further evaluation.
Technology Overview
Cathodic corrosion for producing nanoparticles was (re)discovered when trying to control the electrochemical etching of a scanning tunneling microscopy (STM) tip. We have shown that the metal nanoparticles (NPs) and their alloys can be easily produced by using cathodic corrosion and their sizes and compositions can be controlled. The produced NPs were shown to have high catalytic activity and superior to the commercial ones. Since cathodic corrosion is radically different from all other existing methods of NP synthesis, its ability for tuning properties of NPs is still relatively unexplored, and hence improved characteristics are still expected. Given the enormous simplicity and versatility of the method, we believe that cathodic corrosion has unique potential.
This method includes also the inhibition of agglomeration, the scaling up of NP production and the in-situ impregnation of functional nanocomposites (https://pubs.acs.org/doi/abs/10.1021/acsami.7b18105).

 Figure 1. Photographs of the colloidal nanoparticles (NPs) generated by the cathodic corrosion method. (a) monometallic Au, Pt, Ag, Pd, Ir, Cu, and binary PtAu and PdAu alloy nanoparticles (NPs); (b) AgxAu100-x (atomic ratio) nanoalloys with x = 10, 30, 50, 70, and 90.
Figure 1. Photographs of the colloidal nanoparticles (NPs) generated by the cathodic corrosion method. (a) monometallic Au, Pt, Ag, Pd, Ir, Cu, and binary PtAu and PdAu alloy nanoparticles (NPs); (b) AgxAu100-x (atomic ratio) nanoalloys with x = 10, 30, 50, 70, and 90.

Figure 2. Schematic illustration of the ‘comb-electrode’ setup for the cathodic corrosion method . The block diagram of the global design of the setup (a), the complete cell system (b), the electrode feeding component (c) and an enlarged ‘comb’ consisting of 5 electrode pairs (d). A micrometer screw mounted on the comb electrodes was used to precisely adjust their submersion depth (measured from the moment the electrode touches the liquid surface) in the liquid.
Applications
Producing an extensive variety of nanoparticles and their alloys makes this method unique for almost all applications among:
1. Medical
2. Catalysts
3. Optoelectronics
4. Conductive inks
5. Antibacterial applications (low-cost sub-10-nm silver nanoparticles)
Opportunity
Leiden University is looking for industrial strategic partners for (exclusive) licensing of technology and / or further research and development.
Keywords: alloy, antibacterial, catalysis, cathodic corrosion, colloidal, electrochemical methods, high purity, metal, metal alloy, metal nanoparticle, nanocomposite, nanoparticle, nanoparticle production, silver nanoparticle
DNA-Probe for Non-Destructive Chromatin Sequence Extraction (nodeChrose) This technology can select and “pull down” sequence-specific chromatin fragments using a non-destructive, non-denaturing method
This technology can select and “pull down” sequence-specific chromatin fragments using a non-destructive, non-denaturing method
DNA-Probe for Non-Destructive Chromatin Sequence Extraction (nodeChrose)
Background
DNA in eukaryotic cells is folded into chromatin, ie every 200 base pairs or DNA wrap around a core or histone proteins forming nucleosomes. Both the composition and the location of the nucleosomes play decisive roles in determining the organization of the whole chromatin complex. Moreover, differences in the regulation of the genes encoded in the DNA have been attributed to different chromatin configurations, giving organisms a means to activate specific sets or genes producing a selected set of proteins in different organs, while maintaining identical DNA copies in all cells. In fact, any intervention in the regulation of transcription, including activation / silencing or genes, involves not just DNA, but the complex or DNA and histone proteins.
Nucleosomes form a highly variant class. Their specific variable features include their positioning on DNA along the double-helix, and the occurrence of a number of post-translational modifications on DNA and histones. The occurrence of post-translational modifications is highly regulated and different characters are found in different organs. If fact, misregulation of post-translational modifications can be the origin of (epigenetic) diseases that can even be transferred from generation to generation.
The treatment of the (epi-)genetic diseases might greatly benefit from the capability of monitoring and/or influencing the positions and modifications of histones in chromatin. To avail of selected chromatin fragments extracted from the cell with their intact histone endowment and chromatin structure, is one key to the success of epigenetics research
Technology Overview
This technology can select and “pull down” sequence-specific chromatin fragments in a non-destructive way. This allows for highly focused analysis. i.e. zooming in on a single gene, of DNA sequences with their intact histone protein endowment. At the core of this technology is a novel DNA-probe oligomer formulation, and a methodology to use it for efficient non-destructive chromatin sequence extraction (nodeChrose). This formulation returns a high-affinity probe which is specific to chromatin fragments embedding a known DNA sequence, the “target”, which is long enough to be unique in the genome (Figure 1).
In more detail, the probe is an especially designed oligonucleotide with a target-binding sequence at one end, in which some bases are LNA nucleotides (see “further details”, point (a) ), and at the other end is a covalently bound biotin, which selectively binds to streptavidin coated magnetic beads, allowing an easy pull-down of the extracted chromatin fragments (see “further details”, point (b) ).
The extraction process is initiated by the action of suitable restriction enzymes which selectively cut the chromatin chain and expose a short single stranded DNA portion, the “toehold”, where the oligonucleotide probe can at first “land” and bind. Thereafter, a “strand invasion” occurs at the cleaved end of the chromatin fragment (see figure) allowed by the transient opening of the double stranded DNA and promoted by the high affinity of the LNA-modified nucleotides at the oligonucleotide’s end (Figure 2).
Most remarkably, the whole nodeChrose process happens at room temperature. Such conditions are permissive for the purification of DNA-protein complexes under “native conditions”, where protein-DNA complexes are interacting as they are within a living cell, without the need for crosslinking agents, and without damage to the chromatin fragment nucleosome structure.
Details and State of Development:
- This technology was originally developed to enable the use of single-molecule Force Spectroscopy on specific fragments of folded DNA/Histone complexes (chromatin), and as such it was already successfully employed, yielding insight on chromatin’s protein content and characteristics (this is reported in the upcoming scientific publication).

Figure 1: Design of the probe and generalized mechanism for the invasion of the probe into the target. The target is cut with a restriction enzyme creating DNA-toehold of 4 unpaired nucleotides. The probe consists of an 18 base pair overhang (complementary to the target sequence), a DNA hairpin where a modified base can be incorporated (e.g. a biotin), and a stacking sequence that caps the open end of the target. To increase the affinity of the overhang of the probe to the target sequence contains six LNA bases (colored in red). The mechanism of strand invasion can be summarized in four steps: 1) An endonuclease cleaves the target sequence such that a toehold appears. 2) The probe binds the target at the toehold. 3) Fraying of the double stranded target DNA adjacent to the probe drives strand invasion. 4) A stable hybrid between the probe and the target is acquired, which can be further purified by affinity purification.

Figure 2: Experimental flow. First, the sample containing the DNA of interest is cut with a restriction enzyme, creating the toehold. Subsequently, the sequence specific probe containing the ligand for immobilization is added, so probe-target hybridization can occur. After this, the target can be pulled down with magnetic beads, or alternatively, immobilized on a surface for further analysis.
Applications
1. Any scientific research addressing epigenetics: it is highly cost-effective and has unprecedented resolution
2. Diagnosis: what does the chromatin landscape look like for a particular gene? Variations in chromatin composition have been linked to a variety of diseases.
3. Drug lead discovery in the field of epigenetics: once able to purify native chromatin fragments, one has the proper substrate for epigenetic enhancer/silencing factors. These could be used to identify compounds that interfere with such tasks.
Opportunity
This technology is already available for exclusive and non-exclusive licensing for commercial use, or for evaluation. However, should relevant opportunities for co-development present themselves, they would be surely taken into consideration too.
Keywords: human genetics, chromatin, purification, DNA, LNA, nucleic acid, DNA probe, oligonucleotide, single molecule, sequencing
Platinum Metal-Based Anticancer and Antibacterial Drugs Novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents
Novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents
Platinum Metal-Based Anticancer and Antibacterial Drugs
Technology Overview
Researchers at Leiden University have developed novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents. A family of related ligands can be used in the formulation of the compounds, to tune solubility and activity. The compounds are very stable due to the tetradentate nature of the ligands, coupled with a tetracoordinated (d8) metal center.
While these novel compounds are both simple and cheap to synthesize, they could provide an interesting new alternative for platinum-based anticancer drugs (platinum based drugs such as cisplatin, oxaliplatin and carboplatin already have FDA-approval and are used widely). These new compounds are all the more interesting, because micromolar to nanomolar activities have been observed on cancer cell lines that are resistant to cisplatin.
However the activities are not limited to cancer therapy; applications as an antibacterial agent are also possible. The exact activities of the different compounds depend on the entire compound, while the mode of action depends critically on the nature of the metal.
Details and State of Development:
- The compounds have been synthesized and characterized. In vitro and in vivo (mouse model) experiments have been conducted that have confirmed the high potential of the compounds; in vitro experiments have revealed that the cytotoxicity of some derivatives on human cancer cell lines are among the highest ever reported in the literature, while preliminary in vivo experiments in a mouse model show a decrease in tumor size.
Applications
1. Cancer therapy
2. Antibacterial agent
Opportunity
The researchers are looking for partners to take this invention to the next stage, which would be doing experiments to confirm the biological target, mode of action, and refine efficacy in vivo.
Keywords: metallodrugs, bacteria, cancer, pharmaceutical industry, biotech/medical companies
Novel Approach to Conjugate Adjuvants to Live-Attenuated Vaccines A novel adjuvant technology, that amplifies the interaction of the live-attenuated (sporozoite) vaccine with the host immune system.
A novel adjuvant technology, that amplifies the interaction of the live-attenuated (sporozoite) vaccine with the host immune system.
Novel Approach to Conjugate Adjuvants to Live-Attenuated Vaccines
Background
Live attenuated or inactivated organisms are the most efficient and cost-effective vaccines available. In order to reduce production costs and increase immunogenicity or these vaccines, they can be administered together with adjuvanting molecules. Adjuvants increase the potency of the vaccine. However, in live attenuated vaccines, where the vaccine organism may migrate or distribute in the host, co-delivery or vaccine and adjuvant to the same immune cell is challenging. Particularly for organisms which are naturally very low immunogenic, such as malaria parasites, the conjugation of adjuvanting molecules to the outside of the organism would be an ideal solution.
Technology Overview
LUMC researchers have developed a novel adjuvant technology, that amplifies the interaction of the live-attenuated (sporozoite) vaccine with the host immune system. This technology utilises supramolecular chemistry to pre-target the organism and secondly to conjugate the adjuvant and enhance the immune response. Uniquely, this technology can be applied to both wild-type and attenuated sporozoites and does not require genetic modification of the organism. It can also be used to induce or polarize an immune response at any point during the development of the pre-erythrocytic malaria parasites and/or at specific anatomical locations. Research so far has focused on malaria. However, the developed technology is non-specific and has the potential to be used for other single-cell pathogens such as bacteria or multicellular organisms such as helminths.
Opportunity
We are looking for partner (s) for joint research, development and clinical translation of this technology.
Keywords: parasite, malaria, vaccine, molecular imaging, formulation, parasites, fluorescence, schistosoma, hookworm, bacteria
Imaging Tools to Characterise Migration and Interaction of Parasites within their Host A molecular imaging technology that uses a fluorescent/radioactive tracer to label parasites.
A molecular imaging technology that uses a fluorescent/radioactive tracer to label parasites.
Imaging Tools to Characterise Migration and Interaction of Parasites within their Host
Background
With roughly half of the world's population at risk, the treatment and prevention of parasitic infections remains a medical priority. Parasites typically display migratory behavior, disregard anatomical barriers and interact with their host at several levels during their life cycle. Very little is known about the biology that drives the migration and interaction of parasites within their intermediate or definite hosts. A better understanding of this is essential to help improve the design and development of vaccines. Using malaria parasites as an example, LUMC researchers reasoned that imaging-based tracking of individual parasites or different native malaria species can help understand the interaction of these parasites with their host.
Technology Overview
LUMC researchers have developed molecular imaging technology that uses a fluorescent / radioactive tracer to label parasites. Malaria parasites can be labeled either in the mosquito host or under in situ conditions. Next to this labeling, LUMC has developed a complementary software package that allows for quantitative analysis or parasite behavior in tissue. This molecular imaging technology allows for quantification of the behavioral differences between wild-type parasites and radiation, chemically, or genetically-attenuated vaccine derivatives. Feedback from imaging these parasites provides a reference to which vaccine administration routes and future vaccine formulations can be benchmarked. The imaging technology also supports the isolation of distinct behavioral features that could be critical in interaction with the immune system. Because the technology is based on chemistry rather than GMO technologies, it allows for the first time imaging of wild-type parasites including those species for which GMOs do not exist. In addition, the molecular imaging technology has been successfully applied to Schistosoma mansoni and Necator americanus parasites. In addition to an established application in the field of parasites, the developed technology could also be applied to monitor other pathogens eg bacterial infections or colonization models.
Opportunity
We are looking for partner (s) to license the technology for commercialization and / or to fund research into refinement and clinical translation of the technology.
Keywords: parasite, malaria, vaccine, molecular imaging, formulation, parasites, fluorescence, schistosoma, hookworm, bacteria
A Pre-Targeting Approach for Liver Radioembolisation A two-step pre-targeting approach for radiation therapy with increased accuracy which is less prone to toxic side-effects.
A two-step pre-targeting approach for radiation therapy with increased accuracy which is less prone to toxic side-effects.
A Pre-Targeting Approach for Liver Radioembolisation
Background
Radioembolisation is a local form of radiation-therapy that is increasingly used to treat primary liver tumours and metastases untreatable via surgery or chemotherapy. Currently, radioembolisation procedures are performed in two steps: 1) a scout-step which is used to identify (lung) shunting and to optimize dose using the non-therapeutic radiotracer 99m-technecium magroaggregate, and 2) therapeutic-step using rather costly microspheres containing therapeutic radio-isotopes (90-Ytrium or 166-Holmium).The sequential steps are performed as two discrete procedures, usually separated because of logistical reasons by a period of two weeks. While the clinical benefit of this approach has been demonstrated, its high cost and the preclusion of procedure-related toxicity to healthy tissue e.g. lung remains a challenge. Even when using a scout scan, shunting occurs in 10% and results in the displacement of a fraction of the therapeutic microspheres outside of the diseased area, leading to ineffective dose distribution and serious adverse effects such as radiation pneumonitis. Therefore a cheaper therapeutic alternative that provides higher accuracy is needed.
Technology Overview
Firstly, LUMC researchers have used supramolecular chemistry to develop a two-step pre-targeting approach which integrates the scout- and therapeutic-steps in a single procedure. As the therapeutic component specifically targets the diagnostic component, this prevents discrepancies in accumulation. Hence, the dose prediction becomes more accurate and the shunting issue is solved. Uniquely, the chemical interactions chosen favour complex formation within the liver, meaning that the technology is less prone to toxic side-effects due to lung shunting. Secondly, the pre-targeting concept used creates flexibility in the therapeutic radioisotopes that can be used for the procedure. This means more easily produced radioisotopes can be used to help bring down the treatment cost. Further, the flexibility in the use of radioisotopes also allows for the creation of kit-based radioembolisation formulation that can be prepared in the hospital. With that the current therapeutic window of two weeks can be shortened to one of only hours. Thirdly, the supramolecular chemistry used in this invention could also be adapted for use in chemoembolization approaches or for the subcutaneous needle-injection based delivery of a therapeutic dose to isolated lesions. Again, in both these indications the ability to verify the accuracy of the delivery process before administering the therapeutic component is key.
Opportunity
We are looking for partner(s) to license the technology for commercialization to and/or to fund research into refinement and clinical translation of the technology.
Keywords: radiation therapy, theranostics, radioembolisation, pre-targeting, interventional radiology, nuclear medicine, supramolecular chemistry, pharmaceutical industry, biotech/medical companies
Please note, header image is purely illustrative.
Source: Philip Hogeboom, NL - Wikimedia Commons - CC 3.0 Unported (CC BY 3.0)
Imaging Agents that Specifically Target Peripheral Nerves A peptide-based imaging agent that specifically binds to peripheral nerves, suitable for use in fluorescence-guided surgery application.
A peptide-based imaging agent that specifically binds to peripheral nerves, suitable for use in fluorescence-guided surgery application.
Imaging Agents that Specifically Target Peripheral Nerves
Background
Damage to nerves is a common side effect or surgery that can result in loss of function. Improved visualization of nerves in the operating field, for example by using florescence nerve imaging agents, would help the surgeon avoid such an accidental nerve injury. Ideally, such nerve tracers need to be specific for the peripheral nervous system with little or no cross reactivity with other tissues (adipose and central nervous tissue) as this may lead to unwanted side effects. To date there are no nerve tracers in clinical application.
Technology Overview
Research at LUMC, funded by two grants from the European Research Council, has led to the development of a peptide-based imaging agent that specifically binds to peripheral nerves. The imaging agent targets specific markers on the membrane of myelinating Schwann cells and contains a detectable fluorescent imaging label. This is suitable for use in a fluorescence-guided surgery application. The imaging potential of this agent has been validated in myelin producing cells, dorsal root ganglia cultures, and in the myelin sheaths found in peripheral nerves, both ex-vivo and in vivo.
https://www.lumc.nl/org/radiologie/research/MIIGI/imilab/grants/
http://cordis.europa.eu/project/rcn/104526_en.html
Applications
Fluorescence guided surgery could be used to prevent accidental nerve injury and allow surgeons to preserve nerves during complex orthopedic, cardiologic or oncologic interventions.
Opportunity
We are looking for partner (s) to license the technology to and / or to fund research into refinement and clinical translation of the technology.
Keywords: surgery, image guided surgery, imaging, nerves, pharmaceutical industry, biotech / medical companies
Photoactivatable Anticancer Prodrug A novel photo activated anticancer prodrug consisting in a rigidin analogue caged by a ruthenium polypyridyl complex
A novel photo activated anticancer prodrug consisting in a rigidin analogue caged by a ruthenium polypyridyl complex
Photoactivatable Anticancer Prodrug
Background
In oncology non-resectable, non-metastasized tumor treatment currently relies on chemotherapy, radiation therapy, or photodynamic therapy (PDT). Chemotherapy can be effective but it can be done because of side effects (neuron damage, pain, fatigue, etc.). Radiation therapy and PDT are more local and lower systemic toxicity but they require dioxygen in the irradiated tissues to be efficient. Hypoxic tumors, subset or tumors with high volume or hypoxic, poorly vascularized tissues, low prognosis for the patient.
The present invention is a form of photoactivated chemotherapy (PACT) that combines the advantages of well-defined target (PDT) and PDT (local activation by light and lower side effects). It is more specifically aimed at treating hypoxic tumors.
Technology Overview
The technology relies on the photochemical breakage of a chemical bond. In the prodrug form) a toxic, thioether-containing microtubule polymerization inhibitor is coordinated to a non-toxic ruthenium (II) caging complex. In the dark, the coordination bond between Ru 2+ and the sulfur atom is stable, but under light irradiation is broken, and the non-toxic caging group.
Details and State of Development:
- Collaboration between Leiden University, NL, and Texas State University, USA.
- Uncaged microtubule inhibitor patented by Prof. Alexander Kornienko from Texas State University, USA
- Synthesis, photochemistry, dark stability, and microtubule polymerization inhibition upon light irradiation, have been demonstrated
Chromatography-free synthesis available
- Low dark toxicity and high toxicity in vitro in 2D human cancer cell monolayers demonstrated both in normoxic (21% O 2 ) and hypoxic (1% O 2 ) conditions
- Low dark toxicity and high toxicity in vitro in A549 lung cancer 3D tumor spheroids
- Preliminary results in vivodemonstrate 30% tumor volume reduction under green light irradiation in A549 lung tumor xenografts in nude mice, and no toxicity in the dark, after intraperitoneal injection at 1 mg / kg and with a green light dose of 38 J / cm 2 .
Applications
Market for photoactivated chemotherapy includes brain, liver, head and neck, non-melanoma skin, and eye cancer (Market study available). Best application for tumors with a high ratio of hypoxic to normoxic volume, for which currently available therapies (PDT, radiation, surgery) do not work or are impossible (non-resectable tumors).
Opportunity
The researchers are looking for partners in the field of research, which would be extensive in vivo (mouse model) studies.
Improved Superconductivity with Periodic Nano/Micro Patterning Improving superconducting materials resulting in a controlled modification of the phononic and electronic structure of thin films
Improving superconducting materials resulting in a controlled modification of the phononic and electronic structure of thin films
Improved Superconductivity with Periodic Nano/Micro Patterning
Background
Superconductors are materials that can conduct electricity without resistance. Their superconducting behavior is observed at and below very low temperatures (Transition Temperature, TC), generally under cryogenic conditions; all economically relevant superconductors have cooled down far below the boiling point of liquid nitrogen. Conventional superconductors such as NbTi, NbSn3, Nb, and All are used in sensing applications and magnets for nuclear magnetic imaging instruments. However, the costs of cryogenic cooling make most technologies based on superconductors very expensive and a more widespread application. Researchers at Leiden University have invented a method to improve superconductivity in these and other materials.
Technology Overview
This technology is based on a new approach to engineer/improve superconducting materials: deliberate alterations of the mesoscale structure of the material are realized by using nano and microfabrication techniques, resulting into a controlled modification of the phononic and electronic structure of thin films. This allows the coupling of the electrons with the phonon modes at higher temperatures, with the consequent formation of Cooper pairs and the onset of supercurrents. The physics model that underlies this technology shows how such periodic structures have to be designed to best improve superconductivity (Figure 1) (available at arxiv.org/abs/1704.06805).
Details and State of Development:
- While the fabrication technology is well established and the underlying physics model has passed through peer-review scrutiny, its experimental validation and optimisation are ongoing at the moment of writing.

Figure 1: Possible fabrication methods and realizations. a. Modern nanofabrication tools allow to make periodic patterns. b. Different shapes are possible. c. Different layers of (insulating) materials on top of the thin films have different effects. d. Stacking allows for 3D materials. e,f. Smaller patterning are possible using Moire engineering or single atom manipulation.
Applications
1. Superconducting wires in MRI and NMR instruments.
2. Sensors, including for astronomy and superconducting quantum interference devices (SQUID).
Opportunity
The technology is available for licensing (for commercial use or for evaluation and / or co-development).
Keywords: superconductors, transition temperature, nanopatterning, phononic structure, electronic structure, NMR, MRI
Nanocapillary Electrokinetic System for Particle Tracking / Counting and Microscopy An affordable platform capable of detecting, tracking, counting, and measuring single nanoparticles, vesicles, and biomolecules
An affordable platform capable of detecting, tracking, counting, and measuring single nanoparticles, vesicles, and biomolecules
Nanocapillary Electrokinetic System for Particle Tracking / Counting and Microscopy
Technology Overview
Tracking the motion of single nanoparticles in liquid solution is a gateway to high accuracy particle counting as well as understanding and monitoring physical, chemical, and biological processes at the nanoscale. This technology has recently been demonstrated to carry out high-speed tracking of nanoparticles and macromolecules using elastic light scattering.
The weak scattering or single small viruses (26 nm) was successfully detected. For the first time, their fast thermal diffusion was tracked at a frame rate of more than 2 kHz (see Figure 1). As a step forward towards clinical applications, single urinary vesicles as small as 35 nm were also tracked by elastic light scattering (the first successful attempt of detecting biological vesicles that are narrower 70 nm in freely diffusing suspension). These vesicles have a low-refractive index (n <1.4), as confirmed by comparing their thermal diffusion and light scattering cross section.
This particle-tracking system embeds a silica-based single-mode optical fiber with a hollow-core (nanometer scale) to suppress the free-diffusion or single nanoparticles and direct them into the detection volume. When light is coupled to the nanoparticle-filled optical fiber and detection is performed with a microscope lens at a right angle to the guided illuminating light, the untethered motion of nanoparticles can be imaged and tracked in a quasi-1D geometry for an unlimited unlimited duration with negligible disturbance (Figure 1). For details see ACS Nano, 9 (12), 12349–12357, Open Access.
 Figure 1: a. Schematic representation of our single nanoparticle / virus tracking setup. b. CCMV virus that we have tracked. c. The nanofluidic access or single-mode optical fiber. d. Quasi-1D tracking or a single CCMV virus.
Figure 1: a. Schematic representation of our single nanoparticle / virus tracking setup. b. CCMV virus that we have tracked. c. The nanofluidic access or single-mode optical fiber. d. Quasi-1D tracking or a single CCMV virus.
Applications
1. Biomolecular research
2. Biomedical diagnostics
3. Colloid chemistry
4. Environment pollution
5. Chemical identification
Opportunity
This invention is a user-friendly, plug-and-play add-on device for conventional optical microscope or method to tackle biologically relevant questions based on single nanoparticle detection. The name of this device is 'nanoCET' (nano Capillary Electrophoretic Tracking) and encompasses a cartridge and a stage. Keeping the needs of the biomedical research into account, the nanoCET cartridge will be disposable and cost-effective, and can be detachable from the nanoCET stage. The nanoCET stage is an add-on to the conventional microscope, which can be rented, leased, and purchased as a one-time investment. Interested parties can perform test experiments in the inventor's lab with the resident existing setups.
Keywords: particle count, nanoparticle, microparticle, particle tracking, flow cytometry, microparticle analysis, airborne particles, water analysis, vesicles, exosomes, colloids, liquid chromatography, microscopy, hollow fiber
Single Atom Graphene Nanogap A method yielding a device separating two single carbon atoms from two individual conducting graphene layers
A method yielding a device separating two single carbon atoms from two individual conducting graphene layers
Single Atom Graphene Nanogap
Technology Overview
Chemists at Leiden University have developed a method yielding a device separating two single carbon atoms from two individual conducting graphene layers.
The invention allows the fabrication of a graphene nanogap using a simple methodology. It has been impossible until now to manufacture a graphene nanogap.
The next stage of improvement includes (i) to define the (bio) chemical sensitivity of the device, (ii) chemical edge passivation and determination of the utility of the device as a spectroscope for graphene (and other 2D crystal edge characterization), ( iii) attempting the translocation and detection of individual (bio) (macro) molecules, and (iv) designing the micro / nanofluidic platform (for 'controllably' delivery of a single biomolecule to the point contact; for sequencing or characterization of molecules ).
Details and State of Development:
- 3D Proof of concept (prototype)
- Fabrication process of the gap is validated
Application
1. New tunneling spectroscopes to characterize atomic contacts between two carbon atoms
2. Study nanoconfined media such as gas, liquids, solid
3. Detection of molecules in motion
4. Sequencing molecules
5. Characterization of polymers
6. Study electrical transport through single (organic , bio-organic) molecules
Keywords: biomolecule detection, sequencing, spectroscopy
Concord: Lifesaving Care for Preterm Babies Improved by Keeping the Umbilical Cord Intact An innovative resuscitation table enabling all care needed to stabilize a preterm baby while the umbilical cord remains intact.
An innovative resuscitation table enabling all care needed to stabilize a preterm baby while the umbilical cord remains intact.
Concord: Lifesaving Care for Preterm Babies Improved by Keeping the Umbilical Cord Intact
Background
Every year, 15 million infants are born preterm worldwide. Preterm birth is responsible for over 1 million deaths each year due to complications at birth, many survivors suffer from long-term disability, including learning problems, cerebral palsy or chronic lung problems.
Most preterm infants breathe insufficiently at birth, the cord is clamped immediately to not delay the respiratory support they need to survive. However, immediate cord clamping compromises the infants' cardiovascular function, which can injure its immature organs. Waiting with cord clamping until the infant has been stabilized potentially reduces complications at birth, long term disabilities and mortality.
Technology Overview
With Concord, delayed cord clamping for preterm babies requiring lifesaving care will now be a safe option. Concord is an innovative resuscitation table that makes it possible for the neonatologist to provide all care needed to stabilize the baby, while the umbilical cord remains intact. Concord has an adjustable support bed that can be placed closely above the mother, on which the baby can be placed safely immediately after birth, to keep the sometimes very short umbilical cord intact. In addition, Concord keeps the baby close to the mother to allow bonding.
Details and State of Development:
Specialists at Leiden University Medical Center (LUMC) invented Concord and developed a clinical prototype. The prototype of Concord is used for validation of feasibility and safety in a Phase 1 clinical study. Today, 28 babies have been successfully delivered using Concord at LUMC, in the delivery room as well as in the operating room. The results are very promising regarding the feasibility and safety of the workflow, the quality of care for the baby and the very positive feedback from parents.
Concord Neonatal aims to launch a commercial product by the end of 2018. To achieve this goal, Concord Neonatal is looking for €750,000 in external investment in 3 tranches of €250,000 in 2018, 2019 and 2021.

Benefits
Many cord clamping studies compared immediate cord clamping with delayed cord clamping, focusing on breathing infants:
• Fewer babies needed blood transfusions for anemia - Relative Risk (RR) 0.61
• Reduced risk of bleeding in the brain (IVH) - RR 0.6
• Reduced risk of necrotizing enterocolitis (NEC) - RR 0.62
There is now data available showing another large benefit in delaying cord clamping until ventilation has been established. Results show that waiting with cord clamping until the lung has aerated and the infant has leg stabilized leads to placental transfusion and a more stable oxygenation of the blood and a more stable heart rate during transition. This potentially decreases the injury to the infants immature organs, especially the brains and intestines.
Further Details
Concord Neonatal BV was founded in April 2017 as a spin-out from LUMC, for the development and global commercialization of Concord.
Fabrication of Graphene Nanopore/Nanogap Structures with Implemented Nanofluidic Channel A new technique for the fabrication of nanogap devices which is considerably faster than conventional methods
A new technique for the fabrication of nanogap devices which is considerably faster than conventional methods
Fabrication of Graphene Nanopore/Nanogap Structures with Implemented Nanofluidic Channel
Technology Overview
Chemists at Leiden University have developed a new technique for the fabrication of nanogap devices which is considerably faster than conventional methods and requires low level of fabrication accuracy. It is a platform for the realization of biomolecule detection / sequencing and electron tomography.
The translocation of biological molecules have been detected successfully in nanopores, fabricated in two-dimensional (2D) materials. The mono-atomic thickness of 2D materials is comparable with the spacing between bases composing biomolecules, hence such materials can potentially provide enough resolution for single base identification. The fast translocation of biomolecules - which is not traceable monitoring the ionic current through the nanopore - is an important limitation for the development of nanopore systems for sequencing purposes.
Two graphene electrodes positioned very close to each other with a nanoscale gap in between is a model system to achieve biomolecule sequencing. The electrical current tunneling between the electrodes depends on the geometry and chemical composition of the bases traveling through the gap; fast enough for sequencing.
While conventional nanofabrication techniques have failed to realize nanogap devices in two-dimensional materials so far, the simplicity of our new fabrication technique promises fast development of the devices. High resolution and strong signal to noise ratios are predicted detecting biomolecule bases in nanogap scheme.
Details and State of Development:
- Fabrication process (partly) optimized
- First samples have been measured
Application
1. Biomolecule detection/sequencing
2. Electron tomography
Keywords: biomolecule detection, sequencing, electron tomography
Liposome Drug Delivery Vector Targeting the Blood Brain Barrier A lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle
A lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle
Liposome Drug Delivery Vector Targeting the Blood Brain Barrier
Background
In terms of drug delivery, the BBB is a formidable barrier. Current (pre-clinical) state-of-the-art drug delivery systems, designed to specifically target the BBB, maximally deliver <0.5% of the total injected drug dose to the brain. As such, prognoses for diseases of the brain (eg. Alzheimer’s disease, glioblastoma) remain notoriously poor.
Technology Overview
Researchers at Leiden University have developed a novel lipid, which when mixed with a naturally occurring phospholipid and formulated into 150 nm liposomes, results in a drug delivery vehicle with >10-fold selectivity for the brain endothelium (ie. the BBB) over the systemic endothelium. Encapsulation of both small molecule drugs (doxorubicin) and inorganic nanoparticles (gold nanoparticles) has been successfully demonstrated, resulting in the selective delivery of these reagents to the BBB (following intravenous injection). The novel lipid, required for BBB targeting, is synthesised in a single synthetic step (plus purification) using readily available reagents (<10 Euro/g, Sigma).
Details and State of Development:
The researchers have also demonstrated proof-of-principle of successful encapsulation of small molecule drugs as well as larger cargoes in the newly developed nanocarrier.
Applications
1. Drugs specifically targeting the brain and/or the BBB, such as treatments for strokes, cancer and neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, Huntington’s).
2. Enhancement of brain and/or BBB (theranostic) imaging.
Opportunity
All results have so far been confirmed in the embryonic zebrafish. Validation of this is technology in rodent models is currently ongoing. The researchers are looking for partners from industry/academia/SME to take this invention to the next stage: in vivo testing in mammalian models for brain-related diseas
Keywords: pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
High Throughput 3D Cell Culture Assay An automated method to create 3D ECM-embedded cell spheroids that overcomes previously identified limitations
An automated method to create 3D ECM-embedded cell spheroids that overcomes previously identified limitations
High Throughput 3D Cell Culture Assay
Background
The need for better in vitro screening technology is well known and documented in the field. The currently used 2D cell assays are fast and easy to perform, but lack predictive value. Among the various 3D culture platforms, 3D ECM-embedded cell spheroids most closely represent the in vivo tumor microenvironments. However, several technical hurdles preclude the use of such cultures in high content screening (HCS): i) spheroid creation is difficult and poorly reproducible: ii) it is restricted to certain cell types and iii) spheroid location cannot be carefully controlled, which hampers automated imaging.
Technology Overview
Scientists at Leiden University have developed an automated method to create 3D ECM-embedded cell spheroids that overcomes these limitations (Figure 1). Spheroid formation time is strongly reduced compared to other methods (minutes rather than days) and it can be applied to a broad range of cell types including cells that naturally do not form cell-cell contacts, endothelial cells, various cancer cell lines, and primary tumor cell suspensions. For High Content Screening, they are able to produce 1 spheroid per well in 384 well plates or up to 7 patterned spheroids per well in 96 well plates. Importantly, the spheroids have defined xyz spatial coordinates allowing automated confocal imaging and image analysis algorithms.

Details and State of Development:
- Proof of principle has been established.
Applications
1. Cell culture
2. Tissue engineering
3. High-throughput drug screening
4. Cancer / tumor research
Keywords: 3D cell culture, cell spheroids, high-throughput, drug screening, breast cancer, cancer
Reduction of Antibiotic Resistance-Co-Administration of Food-Grade Compounds Scientists at Leiden University's Institute of Biology have found compounds that reduce antibiotic resistance in pathogenic bacteria
Scientists at Leiden University's Institute of Biology have found compounds that reduce antibiotic resistance in pathogenic bacteria
Reduction of Antibiotic Resistance-Co-Administration of Food-Grade Compounds
Background
The antibiotics market (over 40 billion USD) is hampered by the lack of new (approved) compounds to answer the growth of antibiotic resistance that poses an increasing threat to treat bacterial infections. Only two completely new classes of antibiotics have been introduced over the past 30 years: the oxazolidinone linezolid (Zyvox; Pfizer) in 2000 and the cyclic lipopeptide daptomycin (Cubicin; Cubist) in 2003. Current approaches are focussing on pro-drug strategies, species-specific platforms (identification of (new) specific targets/pathways), and mining untapped sources of natural compounds.
Technology Overview
Scientists at Leiden University’s Institute of Biology have found compounds that reduce antibiotic resistance in pathogenic bacteria.
In the absence of an antibiotic agent, such compounds have no/hardly any bactericidal effect. The compounds are food-grade. Co-administration together with existing drugs will potentiate their effect. This should allow us to re-use antibiotics that have now been abandoned to due to resistance problems.
This current invention allows potentiating existing antibiotics by counteracting antibiotic resistance. While the activity of the compounds is broad, we have established their positive effect in particular for enhancing the efficacy of aminoglycosides and β-lactam antibiotics. The enablement is further helped by the fact that the compounds are food-grade (Figure 1).
Details and State of Development:
- Proof-of-concept. Further development involves identification and/or synthesis of derivatives with similar activity - Co-development of (novel) compounds that reduce resistance to antibiotics.

Fig. 1. Potentiating sensitivity of B. subtilis to antibiotics
Applications
Antibiotic adjuvant strategies: use of natural compounds that reduce resistance to antibiotics.
Opportunity
Currently, the scientists are seeking partnerships for the next stage of development.
Keywords: antibiotics, antibiotic resistance, antibiotic adjuvant
Natural Ionic Liquids and Deep Eutectic Solvents Researchers from Leiden University and colleagues from Delft have identified novel natural ionic liquids and deep eutectic solvents (NADES).
Researchers from Leiden University and colleagues from Delft have identified novel natural ionic liquids and deep eutectic solvents (NADES).
Natural Ionic Liquids and Deep Eutectic Solvents
Background
Researchers from Leiden University and colleagues from Delft have identified novel natural ionic liquids and deep eutectic solvents (NADES). NADES may be used for highly efficient extraction and storage of natural products from plants, such as pharmaceuticals and bio-actives, flavors, natural colorants, etc. Since NADES consistent or simple, cheap, and naturally occurring compounds with a high safety profile, extracts may be used directly in food, pharmaceutical, cosmetic and agrochemical applications.
Ionic liquids (IL) and deep eutectic solvents (DES) consist of two or more solid crystalline compounds that, when mixed together, form a liquid with unique properties. Currently, IL and DES are consistent or mostly toxic, bulky and asymmetric organic cations and are widely used as solvents for industrial processes such as organic synthesis and extractions.
Technology Overview
Researchers from Leiden University and colleagues in Delft have developed a range of new NADES, that consist solely of natural compounds that are normally present in cells, such as certain sugars, simple organic acids and amino acids.
As a proof-of-principle, it was shown that NADES are excellent solvents for extraction of compounds from biological materials which are otherwise difficult to isolate. Examples include plant colorants and drugs, suggesting applicability in drug delivery and the discovery of novel bio-active plant compounds.
NADES can replace existing synthetic ILs and DES that contain toxic compounds and are thus difficult to dispose of. As the NADES constituents are naturally occurring and safe, very simple to make and cheap, many applications can be envisioned including drug delivery, and extraction of various compounds for use in food, cosmetics or pharmaceuticals.
Details and State of Development:
- Proof-of-principle for a number of applications
- Large numbers of combinations for NADES have been characterized
- Extraction and solubility of flower colors, flavonoids and phenolic compounds have been tested
Applications
1. Novel formulations for drug delivery, cosmetics and nutritionals
2. Extraction of new compounds from natural sources
3. Protein or biopharmaceutical stabilization
4. Chemical industry, ionic liquids, cellulose processing and more
Opportunity
Large numbers or combinations for NADES have been characterized. Additionally, extraction and solubility of flower colors, flavonoids and phenolic compounds have been tested.
Keywords: compound extraction, compound solubilization, natural products, drug delivery, biopharmaceuticals
Dynamic Re-usable RAMAN Scattering Sensor This SERS-based sensor allows for dynamical and re-usable measurements and can be used with standard RAMAN equipment
This SERS-based sensor allows for dynamical and re-usable measurements and can be used with standard RAMAN equipment
Dynamic Re-usable RAMAN Scattering Sensor
Background
Surface enhancement in RAMAN spectroscopy has proven to greatly improve the RAMAN signal, increasing sensitivity with several orders of magnitude. Unfortunately, until now, surface enhancement could not be efficiently integrated into most RAMAN-based sensor applications as surface enhancement technology is limited to one shot measurements due to irreversible binding or the analyte to the SERS substrate. This makes SERS technology not suitable for use in applications where continuous dynamic measurements are required.
Technology Overview
Scientists at Leiden University have developed SERS-based sensors that can make dynamic measurements of their environment over time. Sensors like these would be ideal suited for application in, for instance, industrial process monitoring. They can continuously monitor flows of substances (in aqueous solution) for contaminations or formed substances and upon identifying such substances, measure their concentration over time.
Furthermore, this new technology makes the SERS substrates re-usable, which allows for calibration or experimental setups and greatly reduces the number of substrates required per experiment. This makes the new SERSOR very suitable for applications where many different molecules need to be analyzed.
Details and State of Development:
Proof of principle has been obtained, further development is ongoing. We are looking for partners to put commercial products in the market
Applications
1. In-line detectors
2. Process monitoring
3. Medical diagnostics
4. In vivo monitoring
5. Metabolomics and other -omics
Opportunity
Proof of principle has been obtained, further development is ongoing. The researchers are looking for partners to put commercial products in the market.
Keywords: chemistry, sensors, (bio) chemical analysis
Capillary Transfer of Single Droplets Scientists of Leiden University have developed an apparatus to transfer droplets from a first capillary to a second capillary
Scientists of Leiden University have developed an apparatus to transfer droplets from a first capillary to a second capillary
Capillary Transfer of Single Droplets
Background
Transfer ring samples from one capillary to a second capillary is not straight forward. Even more so for small quantities such as droplets.
Technology Overview
Scientists of Leiden University have developed an apparatus for transfer droplets from a first capillary to a second capillary (Figure 1).
For sample handling and sample pre-treatment, the sample is flowing through small tubes in many applications. At Leiden University, scientists had concentrated a sample in a droplet at the end of a capillary (Anal. Chem., 2013, 85 (12), pp 5734–5739). This droplet needed to be transferred to a second capillary for further handling of the droplet (eg transportation to NMR).
Details and State of Development:
- Proof of concept has been established with the working set-up in our lab

Applications
1. LC - NMR
2. LC - EV - NMR
3. LC - SPE - NMR
Opportunity
Leiden University is seeking commercial partners to (co) develop this into commercial products. Applications can be very broad, Leiden University has concentrated its efforts towards sample handling for LC-NMR.
Keywords: analytical (bio) chemistry, LC-NMR, sample transfer
Device to Remove Unwanted Solvents from Biochemical Samples A simple and robust approach to efficiently remove unwanted solvents from liquid mixtures containing dissolved chemical components
A simple and robust approach to efficiently remove unwanted solvents from liquid mixtures containing dissolved chemical components
Device to Remove Unwanted Solvents from Biochemical Samples
Background
Biochemical analysis of complex mixtures is of great importance in various fields of application. A combination of multiple analytical techniques, such as LC-MS, GC-MS and LC-NMR, is often needed to achieve sufficient molecular separation and enrichment. The combination of LC with NMR is not straightforward, certainly not for polar molecules. Scientists at Leiden University have developed an efficient and robust interface between LC and NMR to overcome this problem.
Technology Overview
The exchange of solvent is achieved by the controlled evaporation of a (hanging) droplet using a machine vision feedback loop (Figure 1). The apparatus was successfully tested for a multitude of samples containing volatile, thermosensitive, polar and nonpolar analytes.

Details and State of Development:
- Fully operational system with proof of principle for use in two-dimensional LCxLC and LC-NMR.
Applications
Multidimensional LC
Solvent switching: e.g. NMR
Sample preconcentration
Active component discovery in complex mixtures run in isocratic and gradient systems
The technique can be coupled to spotting techniques such as MALDI-MS and Thin Layer Chromatography
Opportunity
Leiden University is looking for partners for further (joint) development of the device and is looking to license this powerful technology to commercial part(ies).
Keywords: analytical (bio)chemistry, liquid chromatography, NMR
Increasing the Efficacy of Vancomycin: Natural Compounds to Reduce Resistance A compound that decreases the resistance of vancomycin-resistant bacteria
A compound that decreases the resistance of vancomycin-resistant bacteria
Increasing the Efficacy of Vancomycin: Natural Compounds to Reduce Resistance
Technology Overview
Scientists at Leiden University's Institute of Biology have found a compound that decreases the resistance of vancomycin-resistant bacteria.
The sensitizing effect of this compound increases further when the activity of other vancomycin-resistance enzymes are targeted. Their work offers new perspectives for the treatment of diseases associated with vancomycin-resistant pathogens and for the development of drugs that target vancomycin resistance.
Vancomycin is nowadays primarily used to treat serious infections caused by gram-positive bacteria that are known or suspected to be resistant to other antibiotics. Acquired resistance to vancomycin by gram-positive bacteria, mediated through a plasmid-transmittable vancomycin resistance cluster, is a growing problem in the nosocomial environment. Vancomycin resistance evolved in common pathogenic organisms during the 1990s and 2000s, including vancomycin-resistant Staphylococcus aureus (Figure 1).
Vancomycin is a well-known drug against infections with Gram-positive pathogens, the World Health Organization's List of Essential Medicines, a basic health system.
Vancomycin targets the cell wall by specifically binding to the termini of the peptidoglycan precursor lipid II, prior to its incorporation into mature. The resulting weakening of the cell-wall integrity then leads to osmolysis. The termini are universally conserved in bacteria. The Leiden scientists have found the addition of a natural compound to the vancomycin-resistant model organism Streptomyces coelicolor, as well as clinical isolates of Enterococcus faecium dramatically enhanced the efficacy of vancomycin. Fluorescence imaging or S. coelicolor mycelia using BODIPY vancomycin showed that the enhanced vancomycin sensitivity correlated directly to increased binding of the antibiotic to the cell wall.

FIG. 1. Reduction of vancomycin tolerance or S. coelicolor M145 (a naturally vancomycin-resistant model organism)
Details and State of Development:
Proof-of-concept, with further development:
- Study the activity of homologues of the identified inhibitor.
- Screen of novel compounds as inhibitors
- Structural analysis of the interaction between vancomycin resistance enzymes and their inhibitors
- Test efficacy against clinically relevant vancomycin-resistant Enterococci
Applications
Antibiotic adjuvant strategies: use of (natural) compounds that reduce resistance to vancomycin.
Opportunity
Currently, the scientists are seeking partnerships for the next stage of development.
Keywords: antibiotics, antibiotic resistance, antibiotic adjuvant, vancomycin
Mouse Models of Spontaneous Thrombosis A mouse model for VT or AT via “humanizing” mouse coagulation via RNAi of the hepatic antithrombin and protein C genes.
A mouse model for VT or AT via “humanizing” mouse coagulation via RNAi of the hepatic antithrombin and protein C genes.
Mouse Models of Spontaneous Thrombosis
Background
Thrombosis comes in two flavours: VT: Venous thrombosis (with pulmonary embolism as possible results) and AT: Arterial thrombosis (with myocardial infarction or stroke as possible result). Venous and arterial thrombosis are a major source of morbidity and mortality worldwide and both are complex vascular diseases for which pathogenesis is incompletely understood. Animal models are fundamental in our effort to understand the disease and develop better therapy (“Holy Grail” an antithrombotic without bleeding risk as side-effect). Currently there are limited (venous thrombosis) or no (arterial thrombosis) technically reproducible or clinically relevant mouse models for these diseases.
Technology Overview
Researchers at the LUMC have developed a mouse model for VT via “humanizing” mouse coagulation via RNAi of the hepatic antithrombin (Serpinc1) and protein C (Proc) genes.
In addition, they have developed a mouse model for AT via RNAi of Proc in Apoe-/- mice. In initial studies organized and large thrombi superimposed on an aortic root atherosclerotic plaque were observed, a unique and novel finding. This model needs further optimization.

Figure 1: MRI of spontaneous venous thrombosis in a large vessel in the head (mandibular area)

[Figure 2: Arterial (athero)thrombosis in apoliporotein E deficient mouse following RNAi]
Applications
Potential applications would be in preclinical research of venous thrombosis and pulmonary embolism, and arterial thrombosis and myocardial infarction and stroke.
Opportunity
Know-how on the VTE model is available for partnering and/or licensing. LUMC are seeking co-development partners to further optimize the AT model and/or study its response to drugs that are currently used in MI/stroke (lipid-lowering/antiplatelet drugs) and to those in current pipelines (PAR inhibitors, FXI inhibitors, others).
Keywords: animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
Method for Generating PRRS Vaccination Strains Arteriviruses that display reduced translation and function of nsp2TF, as well as the vaccines and immunogenic compositions.
Arteriviruses that display reduced translation and function of nsp2TF, as well as the vaccines and immunogenic compositions.
Method for Generating PRRS Vaccination Strains
Background
Porcine reproductive and respiratory syndrome (PRRS) is the leading threat to the swine industry worldwide. Live-attenuated and inactivated vaccines are now commercially available, but are not without limitations, including concerns on reversion to virulence and insufficient level of protection. The co-existence of different PRRSV strains and subtypes emphasizes the need for cross-protective vaccines. PRRS is the result of infection with a small, enveloped virus (PRRSV) containing a single positive-stranded RNA genome that can be divided into 2 major genotypes: Type I (European) and Type II (North American). Highly pathogenic variants that emerged in China and other Asian countries originated from the Type II genotype.
Technology Overview
The PRRSV genome is about 15kb in length and contains at least 10 open reading frames. Situated in the 5’-proximal region of the genome are the PRRSV replicase genes, ORF1a and ORF1b, which represent nearly 75% of the viral genome. These replicase genes encode long polyproteins that are proteolytically processed into at least 14 nonstructural protein (nsp) products, the largest of which is nsp2. The invention provides the discovery and characterization of arterivirus protein, nsp2TF, the expression of which is dependent upon -2 ribosomal frameshifting at a site located in the nsp2 coding region. This coding region overlaps the portion of ORF1a that encodes the transmembrane region of nsp2 in PRRS and other arteriviruses, including lactate dehydrogenase-elevating virus (LDV) and simian haemorrhagic fever virus (SHFV). Mutations affecting the expression of nsp2TF impair PRRSV replication and result in a smaller plaque phenotype. Provided here are arteriviruses that display reduced translation of nsp2TF and/or altered translation of one or more downstream products, arteriviruses in which nsp2TF function is reduced and/or absent, and vaccines or immunogenic compositions that comprise these arteriviruses. Also provided are diagnostic methods, methods for identifying compounds that inhibit -2 frameshifting, and gene expression tools for eukaryotic systems utilizing -2 frameshifting.
Applications
PRRSV infection in swine is characterized by later term reproductive failure in sows and severe pneumonia in neonatal pigs, and is the most economically significant disease of swine worldwide for the last 25 years. The annual worldwide impact is estimated at over $1 billion. Only a few countries with a representative population remain PRRS-free; the rest of the world is positive and suffers continual reinfections. A recent survey of UK vets estimated the prevalence of PRRS at approximately 50% of all sows and piglets. Improved antiviral therapies for PRRS are necessary.
Opportunity
Available for exclusive licensing
Keywords: arterivirus, PRRSV, swine, pig, vaccine, frameshift, antivirals
Synthetic Control on the Tropism of Cells as a means to Enhance the Efficacy of Cell Therapies Using a pre-targeting approach, abundant membrane receptors on a therapeutic cell can be converted into binders of a multivalent cyclodex.
Using a pre-targeting approach, abundant membrane receptors on a therapeutic cell can be converted into binders of a multivalent cyclodex.
Synthetic Control on the Tropism of Cells as a means to Enhance the Efficacy of Cell Therapies
Background
The degree of engraftment and infiltration of therapeutic cells is thought to be of high importance for the clinical efficacy of cell therapy. At the same time, low engraftment efficacy and survival of implanted cells are reported in clinical applications of cell therapy.In part the grafting can be optimized using local injections, but even then an adhesive strength is required to prevent loss via shunting or diffusion. This intervention provides synthetic control of the cell surface composition using fully reversible non-covalent surface modifications. This technique can be used to convert the cell-tropism for (diseased) cells and tissues of choice. At the same time the technology supports imaging based cell-tracking studies.
Technology Overview
Using a pre-targeting approach, abundant membrane receptors on the therapeutic cells can be converted into binders of a multivalent cyclodextrin polymer. With that a homogeneous cell surface is generated that can be used to introduce a plurality of functionalities using host-guest chemistry. Such functionalities can entail e.g. an imaging label or a targeting vector. In case of the last, the affinity of a cell for other cells or tissues can be manipulated or enhanced.
Details and State of Development:
The researchers are currently looking for collaborators or licensees to further develop this invention.
Applications
Cell therapies in humans are currently limited by the grafting affinity of cells, their viability and diagnostic methodologies that allow monitoring of these processes. The invention provides a solution for these problems. In the generation of 3D cell cultures it is of paramount importance that specific cell-cell interactions can be realized. The presented technology presents a means to artificially control such interactions.
Keywords: cell therapy, cell imaging, biotech/medical companies, tropism, clinical, efficacy, synthetic control
Therapeutic Intervention in Facioscapulohumeral Muscular Dystrophy (FSHD) Novel target mechanisms and in vitro and in vivo models for the development of therapeutic interventions in Facioscapulohumeral Muscular Dystrophy.
Novel target mechanisms and in vitro and in vivo models for the development of therapeutic interventions in Facioscapulohumeral Muscular Dystrophy.
Therapeutic Intervention in Facioscapulohumeral Muscular Dystrophy (FSHD)
Technology Overview
Scientists at Leiden University Medical Center (LUMC), in collaboration with other academic institutions, have discovered and developed novel target mechanisms, as well as in vitro and in vivo models, for the development of therapeutic interventions in Facioscapulohumeral Muscular Dystrophy (FSHD). FSHD is the third most common muscular dystrophy in man with an estimated incidence of 54 per million. Patients suffer from progressive and irreversible weakness and wasting of the facial, shoulder and upper arm muscles. Approximately 20% of gene carriers become wheelchair dependent. There is no cure for FSHD. Scientists at LUMC, in collaboration with other academic institutions, have discovered two novel target mechanisms whereby the two forms of FSHD can arise. The mechanisms represent targets for therapeutic intervention. In addition, cell lines and mouse models of FSHD have been developed and can be used to further research the disease and/or to screen and validate potential therapeutics. The collaborating institutions represent world-leading expertise in the field of FSHD and can also provide ongoing expertise. Partner companies are now sought for research collaborations in this field, and licensing of key technologies available at the institutions.
Details and State of Development:
- Cell models amenable to high throughput screens
- Animal models for research and target validation
Applications
- FSHD Research
- FSHD drug discovery and development
- FSHD gene therapy
- Epigenetic therapy
ApoE3Leiden Mouse The APOE3Leiden mouse displays a human-like plasma lipid profile and is sensitive to diet-induced hyperlipidemia among other illnesses.
The APOE3Leiden mouse displays a human-like plasma lipid profile and is sensitive to diet-induced hyperlipidemia among other illnesses.
ApoE3Leiden Mouse
Background
ApoE deficiency in mice leads to elevated plasma cholesterol levels that are due to the accumulation of remnant lipoproteins, and ApoE deficiency is associated with the development of atherosclerosis. In addition, these mice develop a fatty liver when fed normal chow and show a decreased VLDL-triglyceride secretion. In humans, the mutant ApoE3Leiden isoform is associated with a dominantly inherited form of familial dysbetalipoproteinemia.
Technology Overview
The ApoE3Leiden gene contains a tandem repeat of codons 120 to 126, yielding a protein of 306 amino acids. Transgenic mice expressing ApoE3Leiden develop hyperlipidemia as a result of defective binding of E3Leiden-containing remnant lipoproteins to the LDL receptor and to the LDL receptor–related protein and are susceptible to diet-induced atherosclerosis. Apolipoprotein E (ApoE)-deficient mice develop hepatic steatosis and show impaired very low density lipoprotein (VLDL)-triglyceride (TG) secretion. These effects are normalized with the introduction of the human ApoE3 gene. The APOE3Leiden mouse displays a human-like plasma lipid profile and is sensitive to diet-induced hyperlipidemia, obesity and insulin resistance as well as premature atherosclerosis.
Details and State of Development:
- The ApoE3Leiden mouse has been used in numerous studies over the past decade
- Well validated model
Applications
- Studies for hyperlipidemia, atherosclerosis, obesity and insulin resistance
- Screening compounds for their effects on HDL levels
- Crossbreeding to develop novel mouse models for research use
Opportunity
Researchers at Leiden University Medical Center (LUMC) are interested in research collaborations using the ApoE3Leiden Mouse including crossbreeding to develop novel mouse models.
Keywords: obesity, diabetes, Artherosclerosis, brown fat, metabolic disease
The Development of Therapeutics for CADASIL Patients A potential method for the therapeutic intervention in patients suffering from CADASIL.
A potential method for the therapeutic intervention in patients suffering from CADASIL.
The Development of Therapeutics for CADASIL Patients
Technology Overview
Scientists at Leiden University Medical Center (LUMC) developed a potential method for the therapeutic intervention in patients suffering from CADASIL. CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is a condition causing ischemic brain lesions, which gradually leads to cognitive decline and eventually to dementia. Currently, there is no treatment. The disease is caused by characteristic mutations in the NOTCH3 gene resulting in an unequal number of cysteine residues and misfolding of the NOTCH3 protein. NOTCH3 is exclusively expressed in vascular smooth muscle cells (VSMC) and this misfolding leads to an accumulation of the extracellular domain of the NOTCH3 protein and granular osmiophilic material on the surface of degenerating VSMC. In turn, this leads to impaired vascular reactivity and decreased cerebral blood flow. Scientists at LUMC have succeeded in re-establishing an equal number of cysteine residues in the NOTCH3 protein by the exclusion of specific exons from the mRNA. They demonstrated that this reduces or even delays the accumulation of NOTCH3 on the surface of VSMC. This novel finding could lead to the development of therapeutic strategies for CADASIL patients. Data available on request: Publications,
Non-confidential presentations,
Confidential presentations.
Applications
- CADASIL treatment
- CADASIL research
Opportunity
Partner companies are now sought for research collaborations in this field, and licensing of key technologies. Specifically, the university are looking for companies with a franchise in the treatment of CNS-ischaemic diseases.
Keywords: CNS, CADASIL, gene therapy, rare disease, orphan, exon skipping, oligonucleotides
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers.
Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers.
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank
Technology Overview
Interested in conducting research on obesity and metabolic disease?
Leiden University Medical Center Netherlands has finished enrollment of the Epidemiology of Obesity Study (NEO). Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers in their pursuit of causes and treatments for obesity and metabolic disease. Information ranging from health and depression questionnaires to heart and brain MRIs has been collected from 6,000 participants with a BMI = 27 kg/m2 or higher and 1,000 participants with a BMI < 27 kg/m2. Endpoints include diagnosis of Type 2 diabetes, cardiovascular disease, COPD, asthma, chronic kidney disease, osteoarthritis and all-cause mortality. Analyses were conducted on blood, serum, urine and plasma. Serum, DNA, RNA have been saved for future studies.
Details and State of Development:
Four-year enrollment period is complete; follow-up is on-going.
Applications
Collaborative studies on the effect of obesity on disease Access to data and samples for in-depth studies.
Opportunity
The database/biobank is now open for access. LUMC investigators involved in the NEO Study are also interested in research collaborations using the database/biobank.
Keywords: obesity, metabolomic disease, biobank
New Fixatives with 15-Fold Lower Formaldehyde Concentration Two low-hazardous alternatives to formalin 10% with 10- to 15-fold lower formaldehyde concentration and no change to your histopathology protocol.
Two low-hazardous alternatives to formalin 10% with 10- to 15-fold lower formaldehyde concentration and no change to your histopathology protocol.
New Fixatives with 15-Fold Lower Formaldehyde Concentration
Background
Formalin has stood as the 'gold standard' fixative in pathology labs largely due to its high degree of accuracy, compatibility with downstream histological applications, low cost and ease of use.
Nevertheless, due to the associated health hazards of formaldehyde (1B carcinogen IARC), the search for formalin replacements has been on-going for years, further motivated by the Occupational Safety and Health Administration (OSHA), which encourages its replacement despite the listed benefits . The most well-known alternatives are alcohol-based fixatives, mostly based on ethanol or methanol at concentrations of 50% and higher. However, alcohols do not preserve tissue morphology as well as formaldehyde-based fixatives, the solutions are highly flammable, (neuro) toxic and ethanol possesses the same group 1 carcinogen classification as formaldehyde by the International Agency for Research on Cancer (IARC).
Technology Overview
Leiden University Medical Center and Fix for Life BV have developed two low - hazardous fixatives which require no histopathology protocol adjustments (identical to neutral buffered formalin 10% protocol) and benefit from 10‑ to 15-fold lower concentration of hazardous formaldehyde and better yields and quality of extracted cDNA and RNA. Further PCR experiments are ongoing.
Application
Histopathology fixative for tissue samples
Retractable Needle A needle and sheet system that automatically retracts when contact is made with blood.
A needle and sheet system that automatically retracts when contact is made with blood.
Retractable Needle
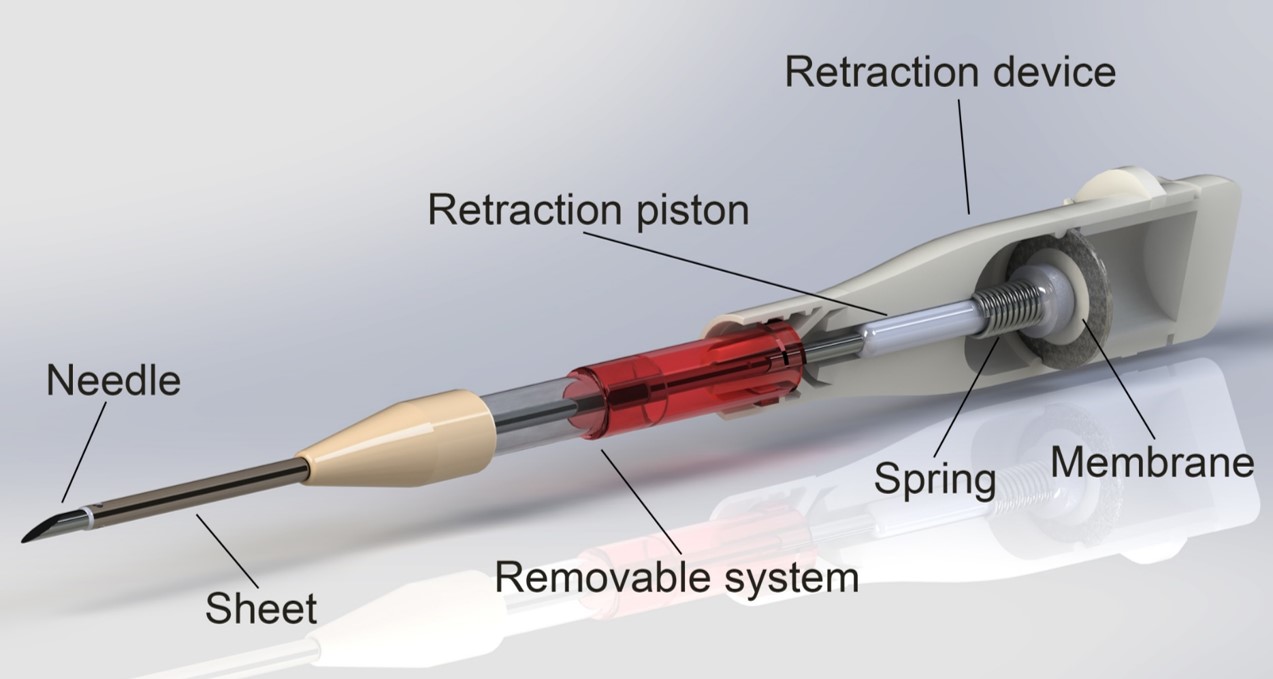
Background
Patients with end-stage renal disease are largely dependent on dialysis as renal replacement therapy. In haemodialysis, blood is passed through a membrane called a dialyzer, or artificial kidney ~3 times a week in order to filter out excess waste and water. The filtered blood is then returned to the body through the second needle. A hematoma (localized bleeding outside of blood vessels) resulting from incorrect needle cannulation if a very frequent problem. Major fistula infiltration occurs with ~5.2% of hemodialysis patient each year. Additionally, incorrect cannulation requires re-cannulation, which is both bad for the vessel, and results in discomfort for the patient. Moreover, 0.4% of all dialysis deaths are due to hematoma (Jose et al, 2017).
Technology Overview
A needle within a cannulation sheet has been designed that automatically retracts to a safe position when contact is made with the bloodstream. When the needle enters the vessel and blood enters the needle, a mechanism is activated which causes the needle to retract ‑ whilst leaving the sheet in place. Therefore, there is no opportunity to puncture the opposing vessel wall.
Applications
- Hemodialysis access cannulation.
- Arterial line placement.
- Pediatric venous cannulation.
Use of 6'-Fluoro-Neplanocin A Derivatives Against Chikungunya Virus Identification of antiviral molecules for the development of therapeutic drugs to treat patients suffering from Chikungunya virus infection.
Identification of antiviral molecules for the development of therapeutic drugs to treat patients suffering from Chikungunya virus infection.
Use of 6'-Fluoro-Neplanocin A Derivatives Against Chikungunya Virus
Background
Chikungunya virus (CHIKV) is an emerging human pathogen that is transmitted by mosquitoes and often causes very severe, debilitating and persisting joint pains, which can last for many months. Since 2006, the virus has re-emerged in an epidemic form and its geographical distribution has rapidly expanded, from Africa to large parts of Asia and South- and Central America. Local outbreaks have also been reported from France and Italy over the past years, due to the presence of the tiger mosquito. Over 6 million people have been affected by CHIKV since its re-emergence in 2006. There are currently no registered vaccines or antiviral drugs available to prevent or treat CHIKV infections.
Technology Overview
Researchers at Leiden University Medical Center have identified 6’-Fluoro-Neplanocin derivatives and related molecules as inhibitors of CHIKV replication in cell culture at submicromolar concentrations (EC50 ~0.2 uM) without apparent cytotoxicity, resulting in a selectivity index of >1000.
Selection of resistant variants and reverse genetics studies demonstrated that CHIKV protein nsP1 is the target of the compound (requiring 2 mutations for resistance). nsP1 is the methyltransferase/guanylyltransferase that is involved in capping of the viral RNA. Biochemical studies confirmed that nsP1 is the target of our compounds. The compounds appear to have a dual mode of action: directly targeting the viral protein nsP1 and inhibiting host SAH hydrolase, causing an indirect inhibition of the unique viral RNA methylation mechanism. The molecules are interesting candidates that might be advanced further into clinical development for the treatment of CHIKV infections.
Stage of Development:
Collaborative project between Seoul National University (Medicinal Chemistry, prof. Lak Shin Jeong) and Leiden University Medical Center (Virology, dr. M.J. van Hemert). The compounds will now undergo ADMEtox analysis and determination of anti-CHIKV efficacy in a CHIKV mouse infection model.
Applications
Development of antiviral drugs for therapeutic use (patients suffering from the persistent CHIKV arthritis) or prophylactic use (in outbreak settings or for travellers to the many tropical and subtropical areas where CHIKV is now endemic).
Reversible Immortalization Method for the Generation of Homogenous Stable Authentic Human Cell Lines Method that allows expansion and redifferentiation of a large population of homogenous, differentiated authentic cells.
Method that allows expansion and redifferentiation of a large population of homogenous, differentiated authentic cells.
Reversible Immortalization Method for the Generation of Homogenous Stable Authentic Human Cell Lines
Background
Researchers at Leiden University Medical Center have developed a broadly applicable method for generating lines or differentiation-competent mammalian (including human) cells. One way to obtain large numbers of differentiated cells from small tissue samples (ie biopsies) is by permanently immortalizing the cells directly after isolation followed by their expansion in a differentiated state and their redifferentiation using specific medium formulations. This, however, rarely yields cells in an advanced state of differentiation (ie authentic cells) due to the continued presence of proliferation stimuli. This method allows massive expansion and subsequent redifferentiation of a cell type or choice through gene transfer and a single-component changes in culture medium composition (Figure 1).
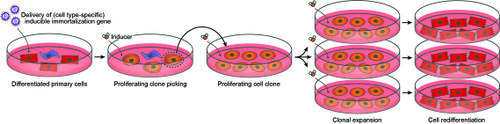 Technology Overview
Technology Overview
This invention concerns the discovery of a new immortalization technique that overcomes many of the shortcomings of the existing immortalization strategies and allows the reproducible generation of large numbers of differentiated cells with properties very similar to those of the cells from which they have been derived (see eg Liu et al. Cardiovasc Res 2018; 114: 1848). These differentiated cells may provide an excellent alternative to cell-based systems relying on differentiation or, for instance, pluripotent (human) stem cells
Applications
- Drug testing
- Therapeutic target identification and validation
- Production of biopharmaceuticals
- Regenerative medicine applications
- Fundamental research
Supramolecular Materials for 3D Cell Culture of Induced Pluripotent Stem Cells and their Derivative Scientists at Leiden University have found a self-assembled squaramide-based tripodal hydrogel for use as a material in cell culture.
Scientists at Leiden University have found a self-assembled squaramide-based tripodal hydrogel for use as a material in cell culture.
Supramolecular Materials for 3D Cell Culture of Induced Pluripotent Stem Cells and their Derivative
Background
Cells that make up tissues are situated within a complex three-dimensional (3D) structure known as the extracellular matrix (ECM). There is growing appreciation for the role the ECM plays in regulating cell behavior and 3D cell cultures (particularly hydrogels) have been developed to allow for closer representation of a cell's natural environment and growth pattern. It is known as the mechanical, structural, and compositional cues, either alone or in concert, drastically altered cell function (Caliari & Burdick, 2016).
The invention describes a self-assembled squaramide-based tripodal hydrogel based on hydrogen bonding and pi-stacking for use as a material in cell culture, particularly for the culture or pluripotent stem cells.
Technology Overview
This technology involves a hydrogel material that can easily be outfitted with various biochemical and biophysical cues that mimic the natural extracellular matrix in vivo for the in vitro culture of cells in 3D.
The inherently modular nature of the materials permits mixing and matching or biochemical and biophysical cues in a user-defined manner. Because the capacity to easily and gently seed and release cells from the matrix, sensitive cell types such as induced pluripotent stem cells and their derivatives can be cultivated within the matrix.
Details and State of Development:
The scientists are currently working on methods to modulate the mechanical properties of these materials to broaden their range in the field of 3D cell culture and exploring a range of peptides for maintenance and differentiation or iPSCs to numerous cell types.
Novel alpha-Glucosidase and alpha-Galactosidase Inhibitors as Antidiabetic, Antiviral Drugs and as Pharmacological Chaperones for Pompe Disease Small molecules that react reversibly or irreversibly with human α-glucosidases depending on nitrogen position of the cyclic sulfamidate.
Small molecules that react reversibly or irreversibly with human α-glucosidases depending on nitrogen position of the cyclic sulfamidate.
Novel alpha-Glucosidase and alpha-Galactosidase Inhibitors as Antidiabetic, Antiviral Drugs and as Pharmacological Chaperones for Pompe Disease
Background
The global α-glucosidase inhibitors market generated $ 4069 million in 2018 and is expected to grow at a CAGR of 2% during 2019-2024. Acarbose, Miglitol, and Voglibose are the most prescribed alpha-glucosidase inhibitors used in the management of hyperglycemia for the treatment of type 2 diabetes mellitus. On the other hand, the combination of small α-glucosidase inhibitors as pharmacological chaperones (PCs) with enzyme replacement therapy (ERT) has shown a synergic effect in improved enzyme activity and reduction of toxic metabolites. Therefore, it is expected that the co-treatment PC-ERT may reduce the amount of ERT necessary to achieve the desired pharmacological effect and therefore may lower lysosomal storage diseases (LSDs) treatment cost. Current ERT is very costly, ranging between € 9 - € 10 million (£ 7.9 - £ 8.8 million, $ 13.0- $ 14. 5 million) during a patient's lifetime. No PC-ERT treatment for Pompe disease exists. Last but not least, recent studies have demonstrated that
ER α-glucosidases I and II are essential for the morphorgenesis of many enveloped viruses, and various iminosugar-based ER α-glucosidase inhibitors has shown in vivo antiviral efficacy in animals infected with Dengue, Ebola and influenza viruses.
Technology Overview
Leiden University have developed a new class of α-glucosidase inhibitors names α-cyclosulfamidates.
α-Glucosidase configured cyclosumaidates react reversibly or irreversibly with diverse human α-glucosidases depedning on the nitrogen position of the cyclic sulfamidate. They have shown that the reversible α-cyclosulfamidate is able to stabilize the recombinant lysosomal human α-glucosidase (GAA) in in vitro and in situ cell experiments and increased enzyme activity is observed in the medium of the cells co-treated with this α- cyclosulfamidate and recombinant GAA. Herein they describe the potential treatment of Diabetes and / or viral infections, as well as the potential of the reversible analogue as stabilizer of GAA for the treatment for Pompe patients in combination with ERT.
Applications
This invention provides a set of new α-glucosidase and α-galactosidase inhibitors. α-Glucosidase inhibitors have shown beneficial effects in multiple applications (antidiabetics, antiviral and as pharmacological chaperones for Pompe disease). Various combinations of enzyme replacement therapy or (mult) gene therapies with a pharmacological chaperone (glycosidase inhibitor) for the treatment of lysosomal storage diseases and glycosidase deficiency related diseases, particularly Fabry, Gaucher or Pompe disease. This combination optimizes the clinical benefit, ie, reduced enzyme treatment, while minimizing disadvantages associated with ERT.
Service offered: Transmission electron microscopy imaging Market sectors: Research and Development
Market sectors: Research and Development
Service offered: Transmission electron microscopy imaging
The electron microscopy Facility at the Leiden University Medical Center (LUMC), offers transmission electron microscopy (TEM) imaging for the analysis of viruses, bacteria, cells, organoids and tissues.
Transmission Electron Microscopy (TEM) provides high resolution imaging for the analysis of all kinds of biological materials, enabling deep insights into the structural details at the nanoscale. TEM supports a wide range of biological samples, ranging from the smallest virus particles, bacteria, cells, to parts of organoids and tissues. The morphology of particles and the inner organization of cells and tissues can be visualized and offers insight. The LUMC has developed automated large scale imaging and visualization tools in combination with supervised machine learning (sML) assisted segmentation of structural features, allowing detection and visualization of structures as well as quantification of structural differences between samples. Imaging is typically performed on plastic embedded and stained sections but can also be performed in 3D using tomography imaging, serial sectioning of serial (SEM) imaging.
test case Vaccine vaccine
Vaccine vaccine
test case

inhiud
bsdhvc
sncjbdc
lknjb
TARGETED COMPLEMENT INHIBITION We have developed strategies to target complement inhibition to specific tissues / cells to achieve local complement inhibition while leaving the systemic complement pool available to fight infections and achieve homeostasis.
We have developed strategies to target complement inhibition to specific tissues / cells to achieve local complement inhibition while leaving the systemic complement pool available to fight infections and achieve homeostasis.
TARGETED COMPLEMENT INHIBITION
SUMMARY
In many autoimmune diseases as well as in transplant rejection, antibodies bind to host tissues and trigger activation of the complement system leading to severe organ damage. The complement system is obviously not there to damage organs but is providing essential protection against infections and mediates tissue homeostasis. This aspect makes therapeutic complement inhibition challenging as strong complement inhibition will result in infectious risk and a dysregulated homeostasis. There are currently a few complement inhibitors on the market, but these inhibitors work systemically, indicating that throughout the body complement is inhibited while the complement activation and tissue damage can be highly localized to one tissue or even one cell type. In addition these drugs are extremely expensive, highly limiting their use. We have developed strategies to target complement inhibition to specific tissues / cells to achieve local complement inhibition while leaving the systemic complement pool available to fight infections and achieve homeostasis.
BACKGROUND
Current treatments for autoimmune diseases and transplantation typically involve (systemic) immunosuppression. In addition, in several diseases where a clear and essential role for complement was observed, complement inhibitors are employed. These complement inhibitors do inhibit complement effectively, however, they require high dosing and come with a risk for infections and with extreme costs. While many more diseases would be eligible for complement inhibitory treatment this is currently not seriously contemplated as it will not outweigh the risks or the costs. By achieving localized complement inhibition with long lasting inhibitors one could circumvent the downsides of current technologies and open up possibilities for many more clinical conditions that can benefit from a localized and short term complement inhibition. So far the only method to achieve local complement inhibition in humans has been local injection in the eye. Clearly not the most sophisticated way to achieve such inhibition and not applicable to other target organs / tissues frequently affected by complement mediated damage.
TECHNOLOGY
The invention builds on the use of bi-specific antibodies that allow targeting of a specific tissue or cell type and provides local complement inhibition on that target. The modular nature of these bi-specifics allows for a wide range of targets to be selected and allows for a inhibition of the classical and lectin pathway or the alternative pathway, or all three.
VALUE PROPOSITION
The complement-targeted therapeutics market is expected to strengthen its roots in the global market at a strong CAGR of 22.9% between 2022 and 2032 (www.futuremarketinsights.com).
TEAM
Dr. Leendert Trouw currently holds a position as Associate professor at the department of Immunology at the Leiden University Medical Center in Leiden/ The Netherlands.
Service Offered: High-Throughput Profiling of Protein N-Glycosylation Employing Linkage-Specific Sialic Acid Esterfication The team of Professor Manfred Wuhrer at Leiden University Medical Center (LUMC), have developed a rapid, robust and linkage-specific high-throughput method for sialic acid stabilization and analysis of complex glycoprotein mixtures such as human plasma N-glycome.
The team of Professor Manfred Wuhrer at Leiden University Medical Center (LUMC), have developed a rapid, robust and linkage-specific high-throughput method for sialic acid stabilization and analysis of complex glycoprotein mixtures such as human plasma N-glycome.
Service Offered: High-Throughput Profiling of Protein N-Glycosylation Employing Linkage-Specific Sialic Acid Esterfication
Market sectors: Research, Biomarker Discovery, Glycosylation
Analysis, Patient stratification
The team of Professor Manfred Wuhrer at Leiden University Medical Center (LUMC), have developed a rapid, robust and linkage-specific high-throughput method for sialic acid stabilization and analysis of complex glycoprotein mixtures such as human plasma N-glycome.
Protein glycosylation is an important posttranslational modification associated, among others, with diseases and the efficacy of biopharmaceuticals. Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS) can be performed to study glycosylation in a high-throughput manner, but is hampered by the instability and ionization bias experienced by sialylated glycan species. Stabilization and neutralization of these sialic acids can be achieved by permethylation or by specific carboxyl group derivatization with the possibility of discrimination between α2,3- and α2,6-linked sialic acids. However, these methods typically require relatively pure glycan samples, show sensitivity to side reactions, and need harsh conditions or long reaction times. The established method was applied to human plasma, and the analysis of the human plasma N-glycome allows highthroughput detection and relative quantitation of approx. 100 distinct N-glycan compositions with varying sialic acid linkages.
We are offering this service to academics / industrial researchers who
may have an interest in accessing this method and associated expertise within LUMC.
Service Offered: Cell Model of Immune Responses Scientists at Leiden University Medical Center (LUMC), have developed a dendritic cell culture system as a read-out of the immune response and as a replacement for animal testing.
Scientists at Leiden University Medical Center (LUMC), have developed a dendritic cell culture system as a read-out of the immune response and as a replacement for animal testing.
Service Offered: Cell Model of Immune Responses
Immune responses are an important part of the fight against numerous diseases. Vaccine companies are interested in knowing if their development products are likely to yield the desired positive immune response, whilst companies with an interest in autoimmune or allergic diseases are developing products which should reduce the immune response in certain circumstances. Biologics companies must also understand how the patient’s immune response might react to their, potentially antigenic, therapeutics.
This in vitro model is able to generate data that could be invaluable in the very early stages of drug or vaccine development, thus saving companies large sums in developing products which will fail in clinical development. In addition, money and time is saved on animal testing. The model is an in vitro culture system of human dendritic cells (the decision makers in immunological processes) which can be used to investigate the influence of ‘foreign’ molecules on dendritic cell function. Within these co-cultures we are able to detect various effector T cell immune responses (T1, Th2 and Th17) or alternatively, regulatory immune responses (Treg) that have been similarly observed in vivo, underlining the predictive capacity of these assays and its potential to serve as an alternative for animal testing.
We are offering this service to academics / industrial
researchers who may have an interest in accessing this assay system and associated expertise within LUMC.
Tumor Associated Carbohydrate Antigens as Targets for Colorectal Cancer Immunotherapy The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue.
The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue.
Tumor Associated Carbohydrate Antigens as Targets for Colorectal Cancer Immunotherapy
Summary
The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue. Furthermore, it is believed that the markers disclosed may be useful therapeutic targets easily accessible as expressed on cell surfaces directly accessible to therapeutics and can be carried by multiple
proteins, reflecting the overall glycosylation phenotype of the cell, providing a broader tumor targeting strategy.
There was a patent filed recently which covers the workflow of extracting, identifying and analysing these highly specific structures as well as another one that includes the specific TACA structures which were found to be solely present in CRC and not in healthy colon providing a number of promising targets for potential therapy.
Background
Colorectal cancer (CRC) is one of the leading malignancies worldwide with over 900,000 deaths in 20201. Conventional therapeutic strategies include chemotherapy, radiation therapy, and surgery. However, due to poor screening strategies and lack of symptoms in early stages, most cases are detected at an advanced stage, leading to unsuccessful treatment. Unraveling the glycome in cancer is important for the development of immunotherapies for treating solid tumors and the discovery of cancer-specific glycan structures is critical for improving how these cancers are targeted.
Technology
The present invention provides a better understanding of the variation in
O-glycosylation and its association with cell phenotypes. An in-depth
structural O-glycosylation analysis of 26 CRC cell lines was performed.
Additionally, the O-glycosylation signatures of primary cell lines as well as the primary and metastatic colorectal cancers they have been derived from were mapped and compared. Furthermore, glycosylation signatures of paired micro-dissected cancer and healthy mucosa were investigated. The released O-glycans were analysed on a sensitive nano-liquid chromatography coupled to a tandem mass spectrometer using electrospray ionization enabling powerful separation of isomeric species, as well as in-depth structural characterization of the epitopes. The major outcomes were as follows:
- Using transformative technology based on laser capture microdissection allowed for the first time to dissect the cancer cell O-glycome of CRC.
- Highly specific glycan structures were observed in cancer cells from most of the tumor tissue which were not present in healthy control tissue.
Value Proposition
The global colorectal cancer therapeutics market is poised to grow by USD 994.94 million during 2019-2023, progressing at a CAGR of almost 3% (www.businesswire.com).
Team
Prof Manfred Wuhrer currently holds the position as Head of the Center for Proteomics and Metabolomics (CPM) at the Leiden University Medical
Center in Leiden/ The Netherlands. Dr Guinivere Lageveen Kammeijer is
Senior Scientist at the CPM focusing on exploring cancer glycosylation for developing novel therapeutic and diagnostic applications. Dr Katarina
Madunic is Scientist at the CPM specialized in dissecting cancer cell
glycosylation.
Immunomodulatory Capacity and Therapeutic Potential of S.mansoni-Derived Proteins in the Context of Immune Modulation, Asthma and/or Allergy Proteomics analysis on several fractions was performed and identified a number of unique proteins of which some were highly abundant. Identification of unique Breg-inducing molecules may form interesting treatment targets for inflammatory diseases such as asthma or allergies.
Proteomics analysis on several fractions was performed and identified a number of unique proteins of which some were highly abundant. Identification of unique Breg-inducing molecules may form interesting treatment targets for inflammatory diseases such as asthma or allergies.
Immunomodulatory Capacity and Therapeutic Potential of S.mansoni-Derived Proteins in the Context of Immune Modulation, Asthma and/or Allergy
Summary
During chronic schistosome infections, a complex regulatory network is induced to regulate the host immune system, in which IL-10-producing regulatory B cells (Breg) play a significant role. Schistosomal egg antigens (SEA) are bound and internalized by specific murine B cells and induce both human and mouse IL-10 producing Breg cells. To identify Breg inducing proteins in SEA, HPLC-fractionation was applied, showing some active fractions. Proteomics analysis on several fractions was performed and identified a number of unique proteins of which some were highly abundant. Identification of unique Breg-inducing molecules may form interesting treatment targets for inflammatory diseases such as asthma or allergies.
Background
Current treatment of asthma are mainly bronchodilators or inhaled corticosteroids with or without the long-lasting β2-agonists. Daily use of these medications is required, to prevent recurrence of inflammation and bronchoconstriction. In addition to the high therapy compliance, other disadvantages of the commonly used treatment for asthma are possible long-term side effects and the lack of responsiveness in severe, therapy-resistant asthma patients. Certain environmental and health factors increase risk of wheezing or asthma in children, or the pathology of existing asthma. Additionally, neither cure nor preventional therapy for asthma exists, only medication to alleviate symptoms. Avenues that focus on primary prevention have the potential to open a completely new market. Helminths derived molecules represent an attractive source of new immunomodulatory therapeutics/biologicals for use in asthma (or allergy) treatment.
Technology
Some candidate proteins were identified as potentially immunomodulatory in fractions of parasite material with the capacity to induce IL-10 from mouse B cells. These crude molecules also seem to be able to block type 2 innate cytokines from human primary bronchial epithelial cells. They were therefore recombinantly produced using specific separation techniques. One specific candidate molecule has also
been tested against human B cells, whereby it also shows the capacity to
induce IL-10. In addition, these pure molecules will also be tested in bronchial epithelial cells to evaluate their capacity to block respiratory infections, such as rhinoviruses.
Value Proposition
The global Asthma and COPD Drugs Market Size alone was valued at
$32988.7 million in 2020 and is projected to reach $52049.54 million by
2030, registering a CAGR of 4.64% from 2021 to 2030. Asthma is a noncommunicable, chronic inflammatory lung disorder of the airways
(www.alliedmarketresearch.com).
Team
Prof. Hermelijn H. Smits currently holds the position as Professor in 'Host-commensal interactions and Immunemodulation' at the Parasitology department within the Leiden University Medical Center in
Leiden/ The Netherlands. She worked at this invention together with her
team.
Cytotoxicity of Synthesized EPD (EPD-S), A Natural Sesquiterpene Lacton, and its Future Clinical Efficacy The present invention is relating to a new anti-cancer drug, EPD-S. This drug shows on itself, as well as in combination with paclitaxel and cisplatin, strong cytotoxicity against ovarian cancer and other cancer types.
The present invention is relating to a new anti-cancer drug, EPD-S. This drug shows on itself, as well as in combination with paclitaxel and cisplatin, strong cytotoxicity against ovarian cancer and other cancer types.
Cytotoxicity of Synthesized EPD (EPD-S), A Natural Sesquiterpene Lacton, and its Future Clinical Efficacy
Background
Ovarian cancer remains still the leading cause of death of gynecological malignancy, in spite of first-line chemotherapy with paclitaxel and cisplatin. Although initial a favorable response, relapses are common and prognosis stays poor.
A plant, Calomeria amaranthoides of the family Asteraceae, endemic to Australia, has been collected in the Blue Mountains (NSW). Asteraceae are known for their natural active compounds, including sesquiterpene lactones (SL’s) which are known for their potential as anti-cancer agents.
This new anti-cancer drug, Eremophilanolides-1-(10)-11(13)-dien-12,8β-olide or EPD, has been proven a very interesting anti-cancer drug. It has exhibited potent cytotoxic effects towards ovarian cancer and other cancers, like melanoma, sarcoma, colon, thyroid, leukemia, breast, but not towards normal cells.
Technology
Most of the experiments have been performed “in vitro”, using the IC50
tests on ovarian cancer cell lines, but also on other cancer cell lines. A
study with nude mice showed that EPD did better than cisplatin, used as
a positive control.
Since EPD has been synthesized by Syncom, (Groningen) (EPD-S) it hasbeen studied by Oncolines and Oncolines Profiler by The Netherlands Translational Research Center (NTRC, Oss, The Netherlands).
Not only has EPD-S been proven cytotoxic on its own, it also has shown strong synergism with paclitaxel and cisplatin in resistant ovarian cancer
cell lines and other cancer cell lines. EPD, in combination with cisplatin, was studied in cell lines with a BRCA1 mutation. In comparison with
olaparib (medication for patients with BRCA1 mutation), showed EPD-S
much stronger chemosensitivity. EPD –S is blocking the pathway of NF-kβ, with apoptosis as result.
Value Proposition
The global Ovarian Cancer market size alone is expected to gain market
growth in the forecast period of 2020 to 2025, with a CAGR of 9.0% in the forecast period of 2020 to 2025 and will expected to reach USD 2382
million by 2025, from USD 1684.4 million in 2019 (https://www.marketwatch.com/).
Team
Dr Caroline van Haaften is the sole inventor of the technology, and she
currently holds the position as Head of Carocell Nederland B.V.
Targeting ligand-bound (solid-phase) C1q We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q.
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q.
Targeting ligand-bound (solid-phase) C1q
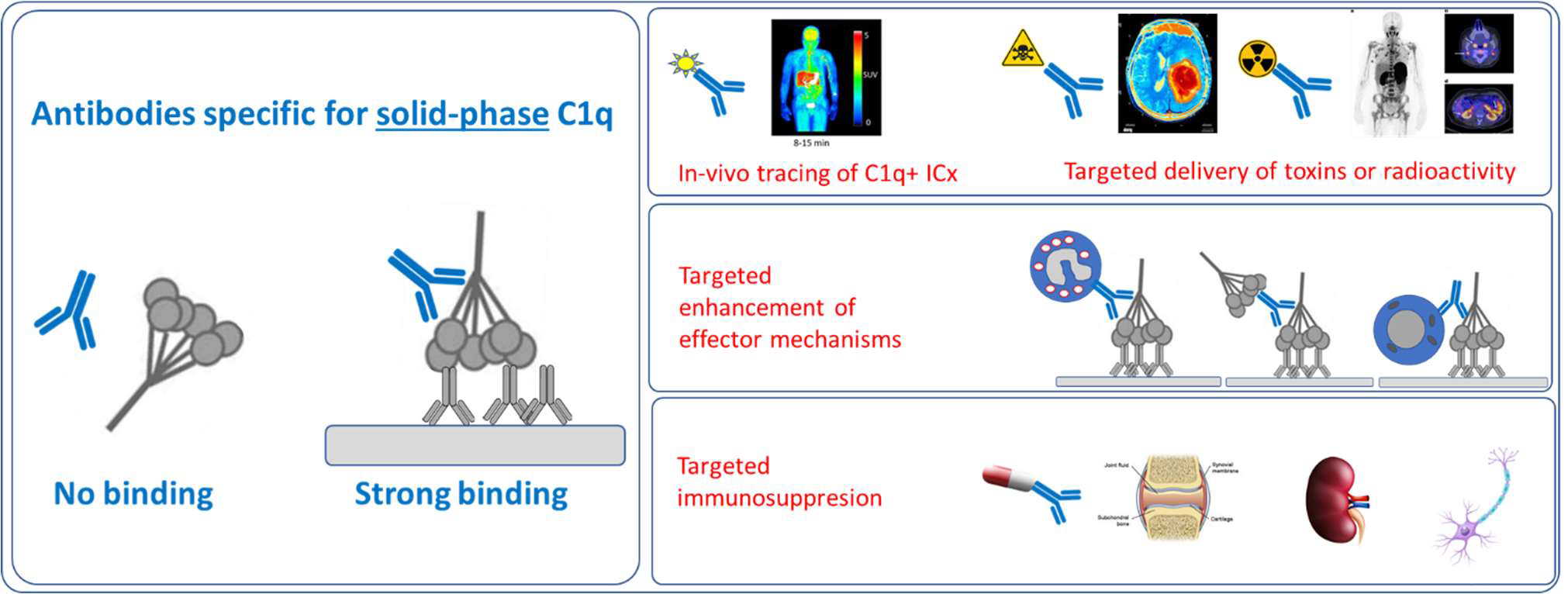
SUMMARY
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q. This property provides unique opportunities as now these antibodies can be used to target C1q in immune complexes or C1q in specific tissues without interference of circulating C1q. Binding to circulating C1q would not only cause a huge sink for any
therapeutics or tracers, but it would also interfere with the normal C1q functions, cause clearance of C1q and reduced complement activity. Using t
BACKGROUND
C1q is the recognition molecule of the classical pathway of complement activation. C1q circulates in the blood at a concentration of around 150 μg/ml. C1q only acquires the capacity to activate the complement system after binding to an array of its ligands. These ligands include for example, immune complexes comprising antigen bound IgG antibodies or antigen bound IgM as well as ligand bound C-reactive protein. If these ligands are present in a sufficiently multimeric format than C1q binds, which results in activation of the C1 enzymes, C1r and C1s and subsequent complement activation takes place. These processes are beneficial in fighting infections and in fighting tumors, but unfortunately do also occur on host tissues where e.g. autoantibodies accumulate. Blocking C1q and classical pathway activity by antibodies has been performed, it depletes all circulating C1q and blocks classical pathway activity, however this works systemically and may put the patient in danger of infections and development of autoimmunity. Here we describe the use of very different anti-C1q autoantibodies, now targeting only ligand-bound C1q while not binding to / interfering with circulating C1q. Such anti-C1q autoantibodies have been described, also by our team, to occur in lupus patients and in some controls. They were shown to be pathogenic, but only if ligand-bound C1q was present in target organs. Obviously, the developed antibodies will be engineered to acquire the desired immune effector properties. Here we describe new, completely human antibodies that we produce recombinantly. This allows us to modify the Fc-domain of the antibody in such a way that it can no longer bind Fc-Receptors or trigger additional complement activity. In this format the antibodies can be safely used as tracers or as antibody drug conjugates. In conditions where one would like to amplify inflammation we can introduce mutations that will enhance Fc-Receptor interactions, that will boost complement activity or that may engage specific immune cells to fight cancer or (antibiotic resistant) infections.
TECHNOLOGY
The invention builds on the use of antibodies that bind to solid-phase C1q. Combined with available technologies we can generate antibodies with inert or active Fc-domains regarding immune activation. In addition we can generate tracers, and antibody-drug conjugates.
VALUE PROPOSITION
The global complement-targeted therapeutics market is expected to
grow at a CAGR of 10.5% from 2022 to 2030. The growth in this
market can be attributed to the increasing prevalence of complement-mediated diseases, rising demand for novel therapies, and growing investments in R&D by pharmaceutical companies.
TEAM
Dr. Leendert Trouw currently holds a position as Professor at the
Department of Immunology at the Leiden University Medical Center
in Leiden, The Netherlands.
Targeted Complement Inhibition We have developed strategies to target complement inhibition to specific tissues / cells to achieve local complement inhibition while leaving the systemic complement pool available to fight infections and achieve homeostasis.
We have developed strategies to target complement inhibition to specific tissues / cells to achieve local complement inhibition while leaving the systemic complement pool available to fight infections and achieve homeostasis.
Targeted Complement Inhibition

SUMMARY
In many autoimmune diseases as well as in transplant rejection, antibodies bind to host tissues and trigger activation of the complement system leading to severe organ damage. The complement system is obviously not there to damage organs but is providing essential protection against infections and mediates tissue homeostasis. This aspect makes therapeutic complement inhibition challenging as strong complement inhibition will result in infectious risk and a dysregulated homeostasis. There are currently a few complement inhibitors on the market, but these inhibitors work systemically, indicating that throughout the body complement is inhibited while the complement activation and tissue damage can be highly localized to one tissue or even one cell type. In addition these drugs are extremely expensive, highly limiting their use. We have developed strategies to target complement inhibition to specific tissues / cells to achieve local complement inhibition while leaving the systemic complement pool available to fight infections and achieve homeostasis.
BACKGROUND
Current treatments for autoimmune diseases and transplantation typically involve (systemic) immunosuppression. In addition, in several diseases where a clear and essential role for complement was observed, complement inhibitors are employed. These complement inhibitors do inhibit complement effectively, however, they require high dosing and come with a risk for infections and with extreme costs. While many more diseases would be eligible for complement inhibitory treatment this is currently not seriously contemplated as it will not outweigh the risks or the costs. By achieving localized complement inhibition with long lasting inhibitors one could circumvent the downsides of current technologies and open up possibilities for many more clinical conditions that can benefit from a localized and short term complement inhibition. So far the only method to achieve local complement inhibition in humans has been local injection in the eye. Clearly not the most sophisticated way to achieve such inhibition and not applicable to other target organs / tissues frequently affected by complement mediated damage.
TECHNOLOGY
The invention builds on the use of bi-specific antibodies that allow targeting of a specific tissue or cell type and provides local complement inhibition on that target. The modular nature of these bi-specifics allows for a wide range of targets to be selected and allows for a inhibition of the classical and lectin pathway or the alternative pathway, or all three.
VALUE PROPOSITION
The complement-targeted therapeutics market is expected to strengthen its roots in the global market at a strong CAGR of 22.9% between 2022 and 2032 (www.futuremarketinsights.com).
TEAM
Dr. Leendert Trouw currently holds a position as Associate professor at the department of Immunology at the Leiden University Medical Center in Leiden/ The Netherlands.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE:
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE:
Summary
Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
Background
HD is a rare neurodegenerative genetic disorder caused by an abnormal expansion of a CAG triplet repeat in the HTT gene. This results in a mutant huntington protein causing the disease. Current approved treatments focus on symptoms rather than the underlying genetic cause. Proteolytic cleavage of the full length protein into smaller and more toxic N-terminal fragments are an important step in HD pathology. Targeting these cleavage sites in HTT RNA directly offers a promising path for altering the disease course, considering the essential roles of the normal huntington protein.
Technology
Our ASO therapy involves splice modulation of HTT pre-mRNA, employing a strategy that targets exon 12 of both the wild-type and mutant HTT. This approach creates a caspase-6 resistant HTT protein (Htt∆12), reducing the toxicity of the mutant protein. This method importantly preserves enough HTT function, crucial for brain health and homeostasis, as complete silencing of HTT can affect motor function, anxiety behavior, and survival.
Research and Development
Extensive preclinical studies conducted in YAC128 Huntington mice have demonstrated the efficacy of the ASO therapy. Key findings include:
- Improved phenotypes: treated mice exhibited significant improvements in body weight, activity levels, and reduced loss of striatal volume
- Gene expression and protein level changes: post-treatment analyses revealed a trend toward normalization of striatal gene expression and protein levels, moving closer to wild-type levels
Value proposition
Our ASO therapy for HD offers a transformative treatment option with potential benefits including:
- Targeted Genetic Therapy: direct targeting of the HTT gene mutation, offering a more effective treatment strategy compared to current symptomatic treatments.
- Preservation of Normal HTT Function: unique exon skipping approach that maintains essential HTT functions, crucial for brain health.
- Strong Preclinical Efficacy: demonstrated functional efficacy in animal models, setting a solid foundation for human clinical trials.
Market opportunity
The Huntington’s disease is a rare disease with prevalence of 7-8 per 100,000 in Europe and North America. There are currently no treatments available that slow or reverse the disease. The HD treatment market is projected to expand significantly, from USD 380 million in 2022 to an estimated USD 2018 million by 2030, at a CAGR of 23.20% (Grand View Research).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team at the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology, ensuring a comprehensive and innovative approach to HD treatment. There are strong links to the Neurology department at the LUMC where dr. Susanne de Bots leads the largest outpatient clinic of the Netherlands with around 500 HD patient visits per year.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE:
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE:
Summary
Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
Background
D-CAA, (Dutch type cerebral amyloid angiopathy (CAA), also known as HCHWA-D or Katwijk’s disease), is caused by a point mutation in the APP gene, leading to brain bleeds and early fatality. Similarly, certain APP mutations and increased production of the harmful amyloid-beta peptide are implicated in AD. Current treatment options are limited, emphasizing the need for targeted genetic therapies.
Technology
The ASO therapy focuses on splice modulation, specifically targeting APP exon 17. This results in an APP protein lacking part of the amyloid-beta peptide sequence, thereby reducing the formation of harmful amyloid peptides.
In vitro studies and Molecular Mechanism
- Initial in vitro studies in human cell lines demonstrated the ASO therapy’s ability to induce exon 17 skipping in the APP gene, leading to an APP protein variant without the amyloid-beta peptide, a key factor in plaque formation.
- Molecular analysis confirmed the ASO's targeted action in altering APP mRNA, showcasing its precision and specificity.
Preclinical Studies in iPSC Models
- Preclinical research in patient-derived human induced pluripotent stem cells (iPSC) showed the ASO therapy's effectiveness in reducing amyloid-beta levels in vitro, a major pathological marker in D-CAA and Alzheimer's Disease, suggesting the therapy’s potential to counteract neurodegeneration.
Safety and Efficacy
- The ASO therapy maintained a favorable safety profile throughout preclinical studies, with no significant adverse effects in treated models showing no overt toxicity at the highest delivered dose.
Value proposition
This ASO therapy targets APP and is applicable to all diseases with harmful amyloid-beta peptide accumulation, such as D-CAA and Alzheimer's Disease, potentially reducing amyloid-beta formation and altering disease progression. Its application extends beyond these conditions, addressing significant unmet needs in various neurodegenerative diseases.
The Alzheimer's disease market alone is expected to grow significantly, with a projected compound annual growth rate of 20.0%, increasing from $2.2 billion in 2020 to $13.7 billion by 2030 (Global Data).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team of the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE

Summary
Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
Background
HD is a rare neurodegenerative genetic disorder caused by an abnormal expansion of a CAG triplet repeat in the HTT gene. This results in a mutant huntington protein causing the disease. Current approved treatments focus on symptoms rather than the underlying genetic cause. Proteolytic cleavage of the full length protein into smaller and more toxic N-terminal fragments are an important step in HD pathology. Targeting these cleavage sites in HTT RNA directly offers a promising path for altering the disease course, considering the essential roles of the normal huntington protein.
Technology
Our ASO therapy involves splice modulation of HTT pre-mRNA, employing a strategy that targets exon 12 of both the wild-type and mutant HTT. This approach creates a caspase-6 resistant HTT protein (Htt∆12), reducing the toxicity of the mutant protein. This method importantly preserves enough HTT function, crucial for brain health and homeostasis, as complete silencing of HTT can affect motor function, anxiety behavior, and survival.
Research and Development
Extensive preclinical studies conducted in YAC128 Huntington mice have demonstrated the efficacy of the ASO therapy. Key findings include:
- Improved phenotypes: treated mice exhibited significant improvements in body weight, activity levels, and reduced loss of striatal volume
- Gene expression and protein level changes: post-treatment analyses revealed a trend toward normalization of striatal gene expression and protein levels, moving closer to wild-type levels
Value proposition
Our ASO therapy for HD offers a transformative treatment option with potential benefits including:
- Targeted Genetic Therapy: direct targeting of the HTT gene mutation, offering a more effective treatment strategy compared to current symptomatic treatments.
- Preservation of Normal HTT Function: unique exon skipping approach that maintains essential HTT functions, crucial for brain health.
- Strong Preclinical Efficacy: demonstrated functional efficacy in animal models, setting a solid foundation for human clinical trials.
Market opportunity
The Huntington’s disease is a rare disease with prevalence of 7-8 per 100,000 in Europe and North America. There are currently no treatments available that slow or reverse the disease. The HD treatment market is projected to expand significantly, from USD 380 million in 2022 to an estimated USD 2018 million by 2030, at a CAGR of 23.20% (Grand View Research).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team at the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology, ensuring a comprehensive and innovative approach to HD treatment. There are strong links to the Neurology department at the LUMC where dr. Susanne de Bots leads the largest outpatient clinic of the Netherlands with around 500 HD patient visits per year.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE
Summary
Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
Background
D-CAA, (Dutch type cerebral amyloid angiopathy (CAA), also known as HCHWA-D or Katwijk’s disease), is caused by a point mutation in the APP gene, leading to brain bleeds and early fatality. Similarly, certain APP mutations and increased production of the harmful amyloid-beta peptide are implicated in AD. Current treatment options are limited, emphasizing the need for targeted genetic therapies.
Technology
The ASO therapy focuses on splice modulation, specifically targeting APP exon 17. This results in an APP protein lacking part of the amyloid-beta peptide sequence, thereby reducing the formation of harmful amyloid peptides.
In vitro studies and Molecular Mechanism
- Initial in vitro studies in human cell lines demonstrated the ASO therapy’s ability to induce exon 17 skipping in the APP gene, leading to an APP protein variant without the amyloid-beta peptide, a key factor in plaque formation.
- Molecular analysis confirmed the ASO's targeted action in altering APP mRNA, showcasing its precision and specificity.
Preclinical Studies in iPSC Models
- Preclinical research in patient-derived human induced pluripotent stem cells (iPSC) showed the ASO therapy's effectiveness in reducing amyloid-beta levels in vitro, a major pathological marker in D-CAA and Alzheimer's Disease, suggesting the therapy’s potential to counteract neurodegeneration.
Safety and Efficacy
- The ASO therapy maintained a favorable safety profile throughout preclinical studies, with no significant adverse effects in treated models showing no overt toxicity at the highest delivered dose.
Value proposition
This ASO therapy targets APP and is applicable to all diseases with harmful amyloid-beta peptide accumulation, such as D-CAA and Alzheimer's Disease, potentially reducing amyloid-beta formation and altering disease progression. Its application extends beyond these conditions, addressing significant unmet needs in various neurodegenerative diseases.
The Alzheimer's disease market alone is expected to grow significantly, with a projected compound annual growth rate of 20.0%, increasing from $2.2 billion in 2020 to $13.7 billion by 2030 (Global Data).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team of the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology.
LAHR: IMPROVED GENOME EDITING ACCURACY AND EFFICIENCY The present invention, termed Ligation-Assisted Homologous Recombination (LAHR), represents an innovative genome editing strategy that combines the genome editing capabilities of CRISPR/Cas12a technology with the high-fidelity of a dual repair mechanism. This method significantly enhances the efficiency and precision of introducing specific nucleotide substitutions while minimizing off-target effects, offering a promising approach for gene therapy and biomedical research.
The present invention, termed Ligation-Assisted Homologous Recombination (LAHR), represents an innovative genome editing strategy that combines the genome editing capabilities of CRISPR/Cas12a technology with the high-fidelity of a dual repair mechanism. This method significantly enhances the efficiency and precision of introducing specific nucleotide substitutions while minimizing off-target effects, offering a promising approach for gene therapy and biomedical research.
LAHR: IMPROVED GENOME EDITING ACCURACY AND EFFICIENCY
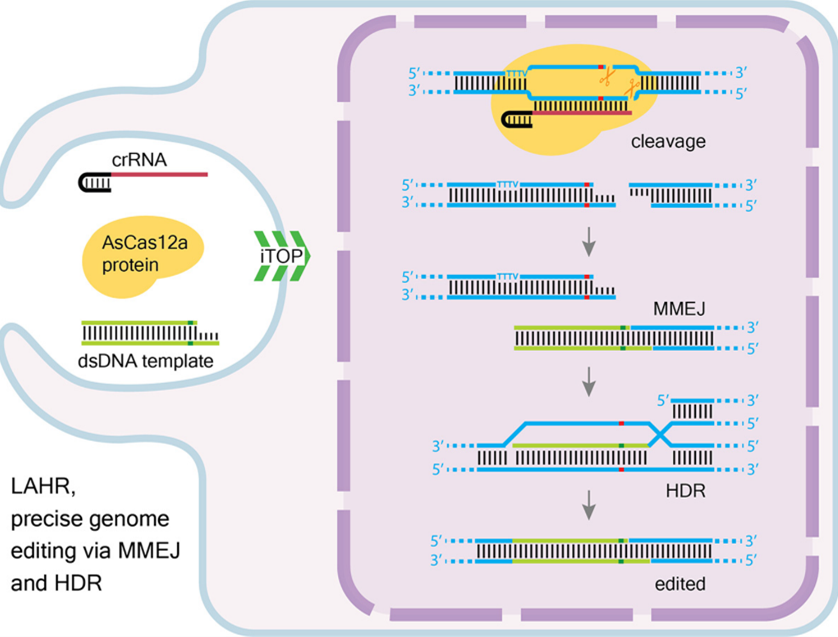
Summary
The present invention, termed Ligation-Assisted Homologous Recombination (LAHR), represents an innovative genome editing strategy that combines the genome editing capabilities of CRISPR/Cas12a technology with the high-fidelity of a dual repair mechanism. This method significantly enhances the efficiency and precision of introducing specific nucleotide substitutions while minimizing off-target effects, offering a promising approach for gene therapy and biomedical research.
Background
Genome editing technologies, particularly CRISPR/Cas systems, have revolutionized molecular biology, providing unprecedented control over the genome. The CRISPR/Cas12a system, recognized for its unique PAM requirements and cleavage properties, generates specific 5′ overhangs, facilitating targeted genome editing. Despite advancements, challenges in editing efficiency and precision persist, particularly when introducing specific nucleotide changes.
Technology
LAHR employs the CRISPR/Cas12a system to create a double-strand break (DSB) with 5′ overhangs near the target mutation. A specially designed double-stranded DNA repair template, containing a complementary 5′ overhang and a short homologous arm, is then introduced. This template engages with the DSB through a resection-independent MMEJ pathway, followed by HDR for target mutation correction. The method has been validated in various cell lines, demonstrating superior efficiency compared to traditional HDR methods, especially in contexts where Cas9 HDR is less effective.
Research and Development
Initial experiments validated LAHR's effectiveness in correcting specific mutations in the EGFP gene within reporter cell lines, demonstrating superior efficiency compared to standard HDR techniques utilizing ssODN templates. Comparative studies further revealed that LAHR matches or exceeds the performance of SpCas9-mediated HDR, especially in scenarios where SpCas9 is limited by PAM sequence requirements.
Investigations into LAHR's mechanics showed the first precise genome editing tool that deploys both the resection-independent MMEJ and HDR mechanisms for precise nucleotide insertion. Additional research highlighted the critical role of homologous arm length and mutation-to-cleavage site distance in optimizing LAHR's editing efficiency.
The technology was successfully applied to introduce precise edits in endogenous genes, including correcting a mutation in the B2M gene and introducing mutations in the ALK and CACNA1D genes, demonstrating LAHR's applicability and efficiency in a real genomic context.
Value proposition
The key value propositions of this innovative technology are:
- Enhanced Precision and Efficiency: LAHR outperforms traditional CRISPR/Cas9 methods in genome editing, offering greater precision and efficiency for accurate genetic modifications.
- Versatility in Application: The technology's ability to effectively target and edit endogenous genes showcases its versatility, making it applicable across various research and therapeutic contexts.
- Overcoming PAM Sequence Limitations: LAHR provides a robust alternative when CRISPR/Cas9 is limited by PAM sequence constraints, expanding the range of genomic targets.
- Dual Repair Mechanisms: Utilizing MMEJ and HDR pathways, LAHR ensures high-fidelity editing, minimizing off-target effects and enhancing the success rate of genetic modifications.
- Potential for Therapeutic Advancements: The precision and adaptability of LAHR hold significant promise for the development of gene therapies, offering new avenues for treating genetic disorders with high accuracy and reduced risk.
The CRISPR and Cas Genes Market, valued at $2.57 billion in 2022, is forecasted to expand at a CAGR of 17.15%, with a value of approximately $9.12 billion in 2030 (Grand View Research).
Team
The development of LAHR technology is led by Professor Niels Geijsen at LUMC, where he leads a team of experts specializing in developmental biology, regenerative medicine, and synthetic biology.
Intellectual property
The LAHR technology is protected by the patent "Method of editing nucleic acid" (PCT/EP2022/084232), which covers its unique genome editing approach. The patent process began in December 2021 and advanced to international filing in December 2022, with national phase entry expected by mid-2024.
Service offered: scanning electron microscopy imaging Market sectors: Research, Drug Discovery and Development
Market sectors: Research, Drug Discovery and Development
Service offered: scanning electron microscopy imaging
The electron microscopy Facility at the Leiden University Medical Center (LUMC), offers scanning electron microscopy (SEM) imaging for the analysis of surfaces and particles.
Scanning Electron Microscopy (SEM) provides imaging for detailed surface and particle analysis of materials at the micro and nanoscale, enabling to gain deep insights into the structure, composition, and properties of their samples. This technique supports a wide range of applications, from manufacturing (drug delivery vesicles) and material science (crystals, alloys, polymers) to biology (pollen, bacteria, organoids, tissues, insects) and environmental research (nano-plastics), helping innovation, improve quality, and data-driven decisions.
Service offered: Lipid nanoparticle analysis using cryo electron microscopy imaging Market sectors: Research, Drug Discovery and Development
Market sectors: Research, Drug Discovery and Development
Service offered: Lipid nanoparticle analysis using cryo electron microscopy imaging
The electron microscopy Facility at the Leiden University Medical Center (LUMC), has developed a workflow for analysis of lipid nanoparticle (LNPs) using automated cryo-electron microscopy (cryo-EM) imaging in combination with supervised machine learning (sML) particle analysis. Using this workflow the size and morphology of EVs can be analyzed, also in 3D.
Lipid nanoparticle (LNPs) are small spherical lipid based particles that are often used as delivery systems. LNP have gained large interest in their use drug and RNA delivery systems in their used for vaccines. LNPs can be synthesized in different ways. Various methods to monitor and evaluate product quality are used during LNP manufacturing, including testing of polydispersity, particle size, drug loading efficiency. Cryo-EM is a valuable high-resolution imaging technique that can directly visualize the structure of the lipid particle and RNA, as well ass contaminants and other particles and therefore can be a valuable additional analysis technique.
Service offered: Extracellular Vesicle analysis using cryo electron microscopy imaging Research, Drug Discovery and Development
Research, Drug Discovery and Development
Service offered: Extracellular Vesicle analysis using cryo electron microscopy imaging
The electron microscopy Facility at the Leiden University Medical Center (LUMC), has developed a workflow for analysis of extracellular vesicles (EVs) using automated cryo-electron microscopy (cryo-EM) imaging in combination with supervised machine learning (sML) particle analysis. Using this workflow the abundance and size of EVs and also other particles (lipoproteins, protein aggregates viruses, etc.) in purified samples can be analyzed.
Extracellular vesicles (EVs) are small (~100 nm) cell-derived membrane-surrounded vesicles that carry bioactive molecules and deliver them to recipient cells. EVs are released from all cells in different ways, have variable lipid, protein and nucleic acid contents, and can have different functions. EVs have gained large interest in their use as biomarkers for several diseases. Generation of highly pure and highly concentrated EV samples from human sources is difficult due to relative low abundancy in combination with overlapping physical properties with other particles (lipoproteins, protein complexes, etc.). Most analysis techniques either do not discriminate between EVs and other particles, or detect a specific marker in EVs. Since cryo-EM is the only technique that can directly visualize the lipid bilayer of EVs, and also can detect contaminants and other particles like viruses, lipoproteins and genomic material, it can be a valuable additional technique for the analysis of purified samples.
Safe structural glass elements
Safe structural glass elements
With this smart and simple solution for connecting structural glass columns and beams, it will become possible to build bigger all-glass structures in a shorter period of time and at lower costs
Glass has become a popular construction material over the last decades, resulting in a doubling of the world glass production. Companies often opt for glass headoffices to underline their corporate image of transparency. In houses and public buildings it allows daylight in, connects the indoors to the outdoors and adds a modern, luxurious feel. Moreover, good double glazing has excellent insulation qualities. But although glass is a cheap building material, glass engineering is expensive, causing the glass building market to be exclusive. Good news: with TU Delft’s novel glass construction system a new era for glass architecture is about to begin.

All-transparent structures
What complicates glass engineering is that glass elements cannot be part of the load-bearing structure; they have to be attached to a brick, concrete or steel frame. This is because it is very hard to directly join glass columns and beams into a structure, as glass cracks unless the load is distributed evenly. In case of point loads (forces applied to a single point, such as a bolt) it breaks unpredictably as a result of the peak stress. TU Delft researchers took up the challenge to design a construction system for safe all-transparent structures that would simplify glass structural engineering and cut building costs. Building on a PhD study on the design of reinforced glass elements with safe failure behaviour, they invented an innovative, simple and almost invisible solution for connecting glass columns and beams, for which a patent is pending.
The invention represents a leap forward compared to other existing concepts. The system allows for a glass-based main load bearing structure with much wider spans, up to thirty meters or more. It is expected to draw much attention of clients and builders, as it is attractive, less expensive, and easy to assemble. For architects of station halls, offices, restaurants, exhibition centres, leisure pools, courtyard roofs, and the like, it opens up new design possibilities. At this moment prototypes of increasing size are built and tested (see photo).
A memristor based on electromigration in a metal alloy
A memristor based on electromigration in a metal alloy
When researching the migration of atoms in a very thin metallic line, nano-scientists of TU Delft encountered a new mechanism for memristor technology. They found that it might be possible to use such a line, a nanobridge, as a memory which can be changed with electrical current.
The type of nanobridge that researchers of TU Delft investigated, possesses characteristics that distinguish them clearly from other metallic contact lines. In metallic lines that are made of one element like platinum or gold the nanobridge is destructed when an electric current is passed through it and atoms start to migrate. The nanobridge shrinks and ultimately breaks near the middle or at the cathode side. The original outer shape of the bridge cannot be restored.

Voids and hillocks
Researchers at TU Delft discovered that a nanobridge which consists of an alloy of palladium (Pd) and platinum (Pt) shows quite a different behavior when a current passes through it and electromigration occurs. Material is transported from the cathode side of the bridge to the anode side. Voids are formed at the cathode side where the atoms move away, while hillocks appear at the anode side.
When the polarity is reserved and cathode and anode switch sides, the atoms move back to the other side, where the areas that were previously depleted are filled with material again. In contrast with a bridge of for example pure gold, which will grow thinner and eventually break, this nanobridge almost returns to its original shape after one full cycle of changing the polarity of the voltage. Nanobridges of the PdPt alloy with various geometrics, for instances a variable width, were tested and each time the same effect of reversible electromigration was observed.
Resistance
When the atoms have migrated, differences in resistance will occur in the bridge. Resistance is higher where voids are formed, while at places where the atoms flock together the resistance will be lower. In this way the change in resistance can be controlled, which is the basic principle of a memristor. To apply the characteristics of this kind of bridge in memristor technology it is best to compare the difference in resistance between the two sides of the bridge. This can be accomplished by putting a metallic contact to the middle of the bridge.
But to really develop this discovery to a method that is fit for large-scale application the number of cycles should be increased to many million times. The researchers tried 15 loops and obtained only a small difference in the shape of the bridge. They expect that transport of atoms in a nanobridge can be repeated many times, but to reach that goal quite a lot of extra research is required. But if this research proves to be successful, a new memristor can be realized.
From Wastewater to sustainable paper sizing
From Wastewater to sustainable paper sizing
Extracellular polymers from granular sludge as sizing agent for paper production
TU Delft is internationally renowned for its groundbreaking environmental biotechnology research. Based on its accumulated knowledge on biopolymers the biotechnology department creates environmentally and economically sound solutions for a wide variety of industries, including the paper industry.
Despite all hopes and expectations, the digital revolution has not resulted in a ‘paperless office’. On the contrary, it is said that the digital media have even caused paper consumption to increase, as people tend to print out the same documents over and over again, instead of keeping the original prints. Unsurprisingly, the paper industry is still one of the largest in the world. It’s a well-known fact that the environmental impact of paper production is significant, as paper mills pollute the air, water and land, while the industry is one of the largest consumers of energy. TU Delft biotechnology researchers have invented a sustainable alternative for a production step that can be quite polluting when using conventional methods: the sizing of paper.

Paper sizing problems
Sizing or size is a kind of starch that is applied to paper to make it water resistant. It is either added to the pulp during manufacturing (internal sizing), or added to the material used for paper coating (surface sizing). It’s an indispensable step in the production of a wide range of paper products used for packaging, wrapping, writing, printing or construction.
In printing paper, for example, sizing prevents the ink from being absorbed into the paper, while improving the paper’s surface smoothness. The problem is that the production of the synthetic polymers used is damaging to the environment and far from carbon-neutral. Besides, they are expensive and not abundantly available. The existing ‘green’ alternative, sizing with algal based alginates, is very effective but too costly to be widely implemented. The solution the TU Delft researchers invented aims to overcome one or more of these drawbacks.
The hidden benefits of granular sludge
The invention is a perfect example of how innovation in one industry can trigger innovation in another. TU Delft is also the inventor of NEREDA, a novel wastewater treatment technology further developed and marketed by Royal Haskoning DHV in collaboration with the Dutch Waterboards, in which purifying bacteria create compact granules with great settling properties. It’s now being used internationally for the sustainable and cost-effective treatment of industrial and domestic wastewater. Until now, the granular sludge produced from wastewater treatment is considered of no further use, so it is disposed of as a waste product.
As the costs of waste disposal represent roughly one third of the wastewater treatment costs, it’s a welcome discovery that extracellular polymeric substances (EPS) obtainable from granular sludge are suitable for paper sizing. EPS are greener than synthetic polymers (as they are obtained from biomass and are biodegradable) and much cheaper to produce, while they have an even higher effectiveness than the much more expensive algal based alginates. Moreover, paper mills will be able to produce the biopolymers from their own wastewater sludge, leading to a zero-emission industry
EPS as sizing agent: advantages in a nutshell
- Cost much less than synthetic polymers or algal based alginates
- Green chemicals (obtained from biomass, biodegradable, renewable)
- Allow for zero-emission industry (paper mills can obtain EPS from their own wastewater)
- Achieve an even better performance than sizing agents using algal based alginates
- Contribute to a higher efficiency of wastewater treatment, as the sludge is recycled
Cheap oscillator delivers top performance
Cheap oscillator delivers top performance
Nearly all electronic instruments need oscillators, from microwaves to smartphones. TU scientists invented a new type of oscillator that is much cheaper, with high performance. Most promising application is in telecommunication chips.
Smartphones need a signal with a constant frequency to be able to communicate with each other. These frequencies are generated by oscillators, electronic circuits that can be compared to the pendulums that make clocks tick regularly. But there’s always a distortion in the signal that is produced. Due to some nature randomness, on a clock one minute doesn’t take exactly as much time as another minute. In electronics the wavelength of the signal fluctuates slightly. A considerable amount of this ‘phase noise’ is acceptable for the higher frequencies (in the range of gigaHerz,) that are used by for example Bluetooth. But for smartphones that use 2G, 3G, or LTE an extremely low phase noise is required.

Low phase noise
Generally, there are two common integrated oscillators. The ‘ring oscillator’ is the cheapest because it takes little space on an chip: approximately 0,01 m2. This costs only about 1 dollar cent. Drawback is the poor performance of this oscillator: phase noise is high. To keep this phase noise as low as possible so far only a more expensive type of oscillator offers a solution. This ‘LC oscillator’ has a much higher performance, but is about 20 times as expensive as the ring oscillator because it takes about 20 times more chip area (0,2 m2).
This oscillator consists of inductor (L), capacitor (C), and amplifier. Both L and C elements can be charged with electric energy. This energy oscillates back and forth very rapidly between inductor and capacitor, which produces a constant frequency signal. Due to resistance of L and C some part of the energy is lost in each cycle. To maintain a constant oscillator, the amplifier compensates this energy loss from a battery.
What makes LC oscillators expensive is the bulky inductor. In the new structure for an oscillator that TU Delft scientists invented, tiny inductors with much smaller chip area are used. This type of inductor has a high energy loss, but this is compensated by using an innovative structure combined with high-efficiency amplifiers. At the same time this results in a very low phase noise. In this new design the inductors and capacitors are connected in series instead of parallel, as is common in LC oscillators. This innovative structure combines the benefits of a ring oscillator and a LC oscillator: low costs and low phase noise with low power consumption. But the invention offers some other great advantages.
Frequency tuning
By adjusting the capacitor the frequency of an oscillator can be tuned. The frequency can be increased or decreased according to the desired application of the oscillator. But with current LC oscillators the frequency range is limited. This new oscillator is capable of producing signals with a wide tuning range of nearly an octave. It means that its maximum frequency is twice the lowest frequency. With normal LC oscillators these values are not possible. Another major advantage of this new oscillator is the extreme fine-tuning of the frequency.
The frequency can be changed digitally with steps as low as just 1kHz, which is ten times better than other oscillators. This fine-tuning is very important for some sensitive electronic circuits, such as digital PLLs. So this new type of oscillator combines the benefits of current oscillators: low phase noise, at low costs, with a comparable power consumption. It is a general oscillator that can be used in several electronic devices. The most profitable application of this oscillator is in wired line or wireless telecommunication chips with markets that produce millions of chips per day.
Advantages
- This oscillator combines low phase noise with low costs.
- Wide tuning range of an octave: maximum frequency is twice the lowest frequency
- Very fine tuning: frequency can be changed in steps of kHz.
- Quadrature signal output.
- Can be used in several electronic devices, e.g. in wired line and wireless communication.
- No further development is needed to be able to use this oscillator.
PowerWindow, a clean energy-producing window
PowerWindow, a clean energy-producing window
Solar panels are cost intensive, have limitations with respect to where they can be integrated into the build environment and, as they are non-transparent, they cannot be used as windows. The PowerWindow converts sunlight into clean energy, using a technique we call ‘BI-CPV’ (building-integrated concentrated photovoltaics). Compared to the failed technology of the ‘80s and ‘90s based on organic dye‘s, our patent-pending inorganic materials have broad-band sunlight absorption and no self- absorption losses. This state-of- the-art technology turns objects through which we have looked through for ages, our windows, into energy source.
PowerWindow is a clean energyproducing tinted window that generates electricity by combining three existing technologies into one. We coat our window with a unique luminescent material. We transport light through the glass to the window edges in the same way as in an optical fiber. Furthermore, at the edges of the window, integrated in the window frames, strip-shaped CIGS PV solar cells convert the light into electricity.


Production
PowerWindow applies a luminescent coating on newly produced windows using a technique called magnetron sputtering. This technique is also used to make tinted windows for cars and offices. Because production is similar to existing techniques and the tinting of the glass is similar, PowerWindow can directly compete with regular office windows, especially because the product has the huge advantage of being able to convert sunlight into clean energy. After the glass is coated, we install the CIGS PV solar cells in the form of strips at the edges of the window in the window frame. These solar cells consist of mainstream thin-film PV technology and can therefore be easily customized and produced to fit many types and shapes of windows.
Future Fit
PowerWindow is a building-integrated technology that enables you to use construction space in a more efficient and environment-friendly way. It contributes to set targets of the European Union towards energy neutral cities. Furthermore, it is a solution that has no geographical limitations. Therefore, PowerWindow fits perfectly in our vision of modern society, infrastructure and global planning. At some point in time we will see electric cars driving on the street when looking through our clean energy-converting windows.
Conclusion
We believe that the PowerWindow is a no-compromise cost-effective sustainable energy solution due to three main reasons. First of all, you do not lose the functionality of your windows and the aesthetics of your building is not affected. Furthermore, you need 100 times less solar cells per surface area, since only strips on the edges of the window are required (instead of the entire surface area). Finally, you are not restricted by the roof surface area to produce clean electricity.
Advantages
- Building-integratable.
- Contributes to EU targets towards energy-neutral cities.
- Thin-film PV technology is easily customized and produced to fit many types and shapes of windows.
- Cost effective because less solar cells are required per surface area and because PowerWindow does not add to the cost of a building as it replaces windows. In other words, it is not an extra shell to a building as solar panels on a roof or facades are.
Silicon transitors on plastic foils
Silicon transitors on plastic foils
Milk cartons with disposable chips which make getting your groceries a lot easier for supermarkets ánd for shoppers. Smartphones with flexible displays and even writing pads with memory chips in each page. New techniques that were developed at TU Delft make it possible to fabricate super-fast and very cheap silicon transistors that can be ‘printed’ on plastic foil or even on textile or paper.
Researchers at TU Delft were looking for cheap ways to produce large quantities of very small and therefore very fast silicon transistors on a plastic foil using a so-called roll-to-roll (R2R) manufacturing process. Currently available R2R processes for producing semiconductors exhibit several problems: the feature size is too big and the alignment between the different layers is not accurate enough. Previous attempts to overcome these obstacles failed: they either cost too much energy or the processing temperature is too high to be compatible with a plastic substrate.
Dewetting
The new method which is developed at TU Delft uses ‘liquid silicon’ or ’silicon ink’ and offers a solution to all these problems. Researchers managed to create semiconductor structures with a very accurate alignment between the layers and with feature sizes smaller than one micron. This is an order of magnitude improvement compared to current roll-to-roll processes for flexible electronics which are limited to feature sizes of a few tens of microns. Very fine patterns are established with a process that is called ‘dewetting’.
Small holes or trenches, formed with microimprinting, are filled with liquid silicon. UV light is used to solidify the liquid silicon by means of polymerization. Exposing the liquid silicon to UV light will cause the ink to spread out against the sidewalls of the holes. The effect is that the lines are further narrowed down, resulting in the desired small features which make transistors that are possibly 100 times faster.
Low temperature processing
The polymerized liquid silicon needs to be transformed into a stable solid state silicon film. Existing methods use a high-temperature hot-plate anneal in order to realize this transformation. Such high temperature anneal however is not compatible with the use of plastic substrates. Researchers at TU Delft found a way to transform the polymerized liquid silicon into solid state silicon without the need for a hightemperature anneal, thereby opening the way to fabricate polycrystalline silicon transistors directly on a plastic substrate.
Smartphones
Chips that are ‘printed’ on plastic foil can be applied in the production of flexible smartphones or in cheap, disposable ID-tags integrated with a sensor. These can be used in for instance supermarkets, which makes paying for groceries al lot easier and faster and creates new possibilities for better stock management.
But the researchers found that the technique they developed for fabricating silicon on plastic can be applied to temperatures well below the processing temperature of well-known plastic foils such as PET. The possibility of fabricating silicon transistors at low temperatures on flexible foils would allow for further applications , for instance a writing pad with a disposable minicomputer on each page.
Advantages
- High speed flexible silicon electronics at low cost
- Very efficient use of energy and material, because processing in vacuum is not required.
- Low temperature silicon processing which makes printing on plastic foils possible
- Compatible with existing roll-to-roll fabrication processes.
Multi Mode Grasper is all-round, smart and intuitive
Multi Mode Grasper is all-round, smart and intuitive
Graspers are of great value to manipulate objects where a worksite is difficult to reach, especially during surgery. However, existing technology requires extensive training to master these skills due to the lack of forcefeedback and non-intuitive use. A TU Delft scientist has succeeded in developing a new grasper that is far more intuitive to use.
Extensive training
A major drawback of current state of the art is the lack of force-feedback that signifies the amount of force being applied by a grasper on an object. The downfall stems from the traditional rigid-linkage design that introduces friction and backlash. As a result, highly skilled surgeons must gain confidence through extensive training or continually exchange instruments to accurately perform sensitive and robust grasping maneuvers. In fact, it has been documented that experienced surgeons rely on alternative signs to interpret the level of forces being applied, for instance, by observing tissue discoloration due to a grasp. This undeniably results in expensive training and operating systems to gain the confidence and skills necessary for surgery.


Sensitive and robust grasping
A TU Delft scientist has invented a solution that resolves the drawbacks of prior state of the art. The result is a multi-mode grasper that offers superior performance through two discrete modes of operation: (1) sensitive and (2) robust grasping. The compliant tool tip design provides continuous visual force-feedback through the deformation of elastic members. Its sensitive force capabilities can be limited for optimal safety during delicate tasks. Its flexible design also improves gripping performance as it conforms more easily around objects, eliminating pinch-points and reducing slippage. Thanks to a contact-aided mechanism, the grasper does not forfeit robust grasping capabilities. By simply advancing an outer sheath, the device is capable of transferring robust forces for rigorous tasks.
Reduction of learning curve
The invention of TU Delft also includes a novel joystick design for manual control of the multi-steerable instrument tip. Whereas a conventional joystick controls only two degrees of freedom (translation forward/backward & left/right), this new joystick-design controls four degrees of freedom (translation forward/backward & left/ right + rotation forward/backward & left/right) with a simple motion of the finger or thumb. Thanks to this new design it is possible to manipulate the 3D position and orientation of the instrument tip independently of the position and orientation of the instrument shaft.
Broad range of applications
Besides surgery, this TU Delft invention has potential for many other applications. Any working enviroment that is difficult to reach and requires manipulation of objects are potential candidates. Space and deep sea mining, government defense, and agriculture are just a few examples of its future applications.
Advantages
- Simple and intuitive to use
- Reduced learning curve, less training required
- Reduced risks for patients
- 2-in-1 grasper performs all tasks, sensitive and robust
- Continuous force-feedback
- Optimal limitation of forces
- Single piece design
- Highly scalable
- No assembly required
- Simple fabrication techniques
- Easy to clean
- Broad range of applications
New method to control release of active substance
New method to control release of active substance
A major drawback of the use of pharmaceutical tablets is that sideeffects may occur due to a too high dosage. A team of TU Delft scientists succeeded in developing a new method to produce pharmaceuticals with a controlled release of the active substance.
TU Delft scientists made use of the principles of Molecular Layer Deposition, which is a gas-phase process to deposit conformal thin films of organic nature. The new method is based on alternatingly providing precursors to a solid surface. These precursors are chosen in such a way that the reaction of the precursors is self-limiting.
Self-limiting
Once the gas precursor is chemisorbed at the surface, there is no further reaction due to the completion of the available surface sites. As a result of this selflimiting characteristic, it is possible to grow thin films, which can be controlled on atomic level. This superb control leads to excellent step coverage and conformal deposition on complex nanostructures. TU Delft scientists successfully applied Molecular Layer Deposition to particles in a fluidized bed (particles suspended in an upward gas flow). Research shows that the dissolution rate of these coated particles decreases with the increase in number of coating cycles, while the uncoated particles dissolve instantaneously.

Tuning dissolution behaviour
This TU Delft invention allows for tuning the dissolution behaviour of the coated particles by adjusting the thickness of the coating. This new method makes it possible to produce pharmaceuticals, that allow for delivery of a stable dosage of active substance. Compared to everyday reality, in which patients are confronted with an overdose of active substance quite commonly, this is a major improvement. Moreover, as this invention allows for a stable delivery of active substance over a long time, another major advantage is that it reduces the number of pharmaceutical doses, which patients have to take.
Easy to implement
Compared to prior state of art, like for instance spraying a liquid on top of the particles in order to create a coating, this invention excels in controlling the thickness of the coating. Moreover, less material is required as there is less waste during the production process. Another major advantage is that it is quite easy to implement, using existing prod
Promising invention
Besides the pharmaceutical industry this invention might have a huge impact in other areas, like for instance fertilizers, herbicides and corrosion inhibition. Besides these areas producers of detergents, like dishwashing tablets, might benefit from it as well.
Advantages
- Less active pharmaceutical ingredients needed
- fluctuating dosage of active substance
- Less byproducts during production
- Easy to implement in existing process schemes
- Compatibility with existing equipment reduces investments
Biobased membrane compund portects contrecte from drying
Biobased membrane compund portects contrecte from drying
Undesired drying of concrete and cement paste is a nightmare for any construction engineer. The consequence of moisture loss and drying is that cracks appear, which results in a dramatically deterioration of the concrete or cement paste surface.
Inspired by the art of molecular cooking a team of TU Delft scientists succeeded in developing a new bio-based membrane compound with unique and highly sustainable characteristics. The method is based on the use of a biobased liquid, which is made of bio-degradable polymer, like for instance sodium alginates. When sprayed on the surface of concrete or cement paste, a rapid chemical reaction takes place that cross-links the polymers with the polyvalent cations from the surface. The result of this chemical reaction is that a gel layer is formed, which excels in a moisture bearing capacity. Tests of TU Delft scientists show that the surface of coated concrete and cement paste is successfully protected from undesired drying.


Robust membrane compound system
Construction engineers and material experts recognize the major disadvantages of the current generation of membrane compounds. At first, the quality of current membrane compounds highly depends on the weather circumstances, as well as on the time of application. The latter is a result of the fact that the organic solvent-based polymers have the intention to diffuse in cement-based material. Secondly, the organic solvents, that are non-bio degradable, can be harmful for the environment. The bio-based membrane compound, developed by TU Delft scientists, can’t diffuse into a cement-based material, but instead, it gellifies at the surface and forms a very protective layer, resulting in an extremely robust membrane compound system that avoids the concrete is drying.
Environmentally friendly
As this method is based on the use of bio-degradable polymers, this invention can be classified as extremely environmentally friendly. As a matter of fact, this method requires far less water than some conventional methods. This contributes to a sustainable society and is a major advantage for usage in areas where water is a scarce resource.
Last but not least, this new method of protecting concrete and cement from drying is very costeffective, as it is based on the use of lowvalue substances, such as sodium alginates. This invention has a huge potential for any line of business that makes use of concrete or cementious surfaces, that have to be cured or protected from moisture loss and drying. Amongst others, both construction industry as well concrete fabrication industry can benefit from this revolutionary innovation.
Advantages
- Protects strongly against moisture loss and drying
- Quality is independent from weather circumstances and time of application
- Environmentally friendly
- Very cost effective
Self healing concrete-materials that can repair itself
Self healing concrete-materials that can repair itself
Prevention is better than cure, and that applies to the construction industry too. Buildings, however, are exposed to the elements for years, even centuries, on end. Air, changes in temperature and, especially, moisture can weaken even the sturdiest of materials over time. However, there is a new generation of materials that can repair itself. That is less expensive than putting up a new building, and better for the environment. Enter the selfhealing concrete.
The inspiration for self-healing concrete comes from nature – limestone producing bacteria, to be specific. When embedded in concrete, these bacteria should be able to repair cracks in it. However, to be able to survive in the concrete, they have to come from good stock: the pH-value of concrete is around 13, which is an extremely alkaline environment. Moreover, the bacteria have to be able to survive the concrete mixer and then they have to wait for years before being able to carry out their restoration work.


Bacillus is best
Bacteria of the bacillus species have exactly the right characteristics. Their spores can survive for decades in a kind of sleep mode, without food or oxygen. In concrete, they will only come to life if water and oxygen are ‘added’ – in other words, if a crack appears in the concrete. They are then able to multiply and produce limestone, thereby closing the crack in a few weeks. Once the crack is closed up completely, moisture can no longer get into the concrete, so it will not weaken. This is the perfect solution for underground spaces for example, in which it is always damp.
How it works
A healing agent for concrete has been developed that is made up of two components: bacillus spores and calcium lactate nutrients. These are set separately into expanded clay pellets, or alternatively in compressed powder granules, a few millimetres in size. The pellets are then added to the wet concrete mix.
When, hopefully years later, cracks begin to form in the concrete, water will enter and open up the pellets. The bacteria will germinate and start to feed on the lactate, thus combining the calcium with produced carbonate ions to form calcite, or limestone. Full scale outdoor testing is under way. A building in the South of Holland has been covered with the bioconcrete and will be monitored over a period of two years.
Flexible Grasper: Perfect Balance between Sensitivity and Force
Flexible Grasper: Perfect Balance between Sensitivity and Force
For surgeons, the ideal grasper can feel what is being grasped when performing precise and robust surgery. A grasper developed at TU Delft offers a perfect balance between sensitivity and force.
Surgeons use graspers in minimal invasive surgery, where instruments are inserted through small incisions in the abdominal wall to diagnose and manipulate tissue. Current graspers are rigid instruments that provide surgeons with very little force feedback, or sensory information about what is being grasped and the forces they are exerting. These instruments lack sensitivity between the actuation handle and the tool tip of the instrument because they consist of separate parts linked together with pinhole joints.
This design introduces backlash and friction that distorts the surgeon’s senses when gripping an organ or tumor. Due to this lack of force feedback, surgeons need extensive training before they are able to perform minimal invasive surgery. Yet, inadvertent tissue damage still occurs in surgery. Highly skilled surgeons develop alternative methods to estimate the forces they apply, for instance, by relying on discoloration of tissue. This skill can take a surgeons career to master, with little standard of consistency

Flexible
A TU Delft scientist has invented a compliant grasper that provides surgeons with a much better sense of what is being grasped. Its single-piece design eliminates friction and backlash, while simplifying fabrication, part-assembly, and sterilization. Another major improvement is the creative use of flexible members within the instrument. As a surgeon exerts force with this grasper, flexible members deform as a function of the applied force.
In this way a surgeon is able to observe the precise forces, further enhancing the feel of what he’s doing: the surgeon gains sensitivity of applied forces. This gives a surgeon better control over the forces he is exerting during precise grasping. Because of the instruments flexible design, the gripping jaws conform better to the shape of the object that is being grasped. This human-like grasping not only reduces slippage, but also further enhances usability through intuitive use.
Robust
The flexible members can be customized to the delicacy of any task. For robust forces, this grasper switches to a more powerful mode. For this purpose, the grasper features an outer sheath that can be moved forward. The tool tip contains shape-locking elements that shift into contact when the sheath is advanced.
This transmits large forces to a precisely formed grip. This feature can be used for fixating tissue, and can be quickly retracted to maintain the sensitivity of the instrument. This new grasper is also useful in other fields where objects are manipulated in difficult to reach places. In deep-sea mining, this device can be useful for distinguishing delicate artefact or hard rock. The same applies for space mining, for instance, the Mars Rover. A flexible grasper that can easily be switched from sensitive to robust modes offers possibilities in applications far beyond minimal invasive surgery
Passive compensation of a tram's stray magnetic fields
Passive compensation of a tram's stray magnetic fields
A 2 kilometer stretch of Delft’s tramline 19 is the showcase of the world’s first compensation system for a tram’s stray magnetic fields, preventing sensitive measurements in TU Delft’s nano physics & electronics labs from being disturbed.
The tram (or ‘streetcar’) is a popular means of public transportation. Compared to buses, trams can accommodate more passengers, they move and load faster and they’re more environmentally friendly. That’s why TU Delft gladly welcomed the construction of a tramline that would connect the university to the city centre and the Technopolis Innovation Park.
When researchers expressed concerns about the tram’s stray magnetic fields, it was decided to look for a solution that would allow TU Delft’s renowned nano research and the convenient tram to peacefully coexist. The win-win outcome was an interference-free tram on campus and a TU Delft patent for the compensation system that made it possible.


Overhead wire segmentation
When a tramcar accelerates, it uses up to a 700 Ampere current, supplied by overhead lines and returning to earth through the rails. It’s this current loop that causes the disturbing magnetic fields - and the bigger the loop, the larger the magnetic field. An existing solution (known from literature) is to minimise the surface loop area, which can be done by bringing the feeding wire and the returning wire closer together.
To realise this, the overhead lines are divided into segments that are insulated from each other, and the feeding line is placed underground between the two tramway tracks. As the overhead wire is charged segment by segment through a vertical cable, the resulting loops can never be bigger than the overhead wire’s height multiplied by the segment’s width. However, this solution does not reduce the fields sufficiently, certainly not at distances from the track comparable to the segment’s width.
Two-way connection
The TU Delft inventors had the additional idea to connect the overhead line segments on both sides to the underground power line and use the relatively high resistance overhead line as a voltage divider. This way a tramcar is fed from two sides of the segment, creating two current loops in opposite directions. When the tram is in the middle of a segment, the two currents are equal, and as the two current loops’ contributions to the total magnetic field at some distance are equal but reversed, they neutralise each other.
When the tram is proceeding towards the end of a segment the stray magnetic fields are also compensated, but here the explanation is a bit more complex. The relative currents in the two loops are inversely proportional to the relative resistance of the overhead line sections that feed the tram. Since the resistances are proportional to the lengths of these sections, the short section provides most of the current. The magnetic field strength is the product of current and surface area, so both loops still give the same, but opposite contribution to the total field.
No local compensation needed
The compensation system is expected to reduce a tram’s stray magnetic fields at 50 meter distance by a factor of almost 10. This will save researchers the trouble of locally generating compensating magnetic fields around sensitive equipment. The invention doesn’t compromise the tram’s power supply security, it doesn’t require adjustments to the tramcars, and it can be built with conventional materials and equipment. As the system is built with passive components only (meaning no electronics are involved), it offers easy maintenance and a long life time.
The compensation system’s advantages in a nutshell
- reduces a tram’s stray magnetic fields at 50 meter distance by a factor of almost 10
- no need of locally generated compensating magnetic fields around sensitive equipment
- doesn’t compromise the tram’s power supply security
- doesn’t require adjustments to tramcars
- can be built with conventional materials and equipment
- easy maintenance and long life time (built with passive components only, no electronics)
BLISP makes mobile devices more energy efficient
BLISP makes mobile devices more energy efficient
Mobile devices with passive radio do not need their own source of energy. However, their range is limited. Using active radio increases that range considerably, but it still requires a battery. TU Delft researchers have developed an integrated system that makes optimum use of the advantages of both platforms by switching between active and passive radio just at the right moment.
Passive radio – also known as backscatter radio – always uses an external source of energy. A good example of this is the OV public transport chip card used in the Netherlands. When you hold the chip card close to the card reader, they make contact with each other, and the reader also transfers energy to the card. This enables the card to share information without requiring a battery. However, this kind of communication has a downside. Over a greater distance, the energy and information transfer no longer works.
It is possible to cover greater distances with active radio but, in this example, the chip card would need to have its own chargeable battery.

Hybrid platform
Researchers in the Faculty of Electrical Engineering, Mathematics and Computer Science at TU Delft were the first in the world to develop a hybrid radio platform concept combining passive and active radio techniques. After all, it would be advantageous if a mobile device could use backscatter over short distances and, when the range is insufficient, automatically switch over to active radio. Switching thus would mean that power consumption would be limited to times when active radio is in use, with the result that the battery would not need to be charged as often.
In order to put this idea into practice, the TU Delft researchers combined state-of-the-art platforms WISP (Wireless Identification and Sensing Platform) and BLE (Bluetooth Low Energy). The former is a passive and the latter an active radio technology. They named the hybrid platform BLISP: a combination of the first and last letters of the two acronyms.
The scientists then developed a protocol to enable both systems to communicate with each other and check whether passive radio is (again) available. The moment of passive radio evaluation is a big part of the system efficiency. Furthermore, the hardware has been constructed in such a way that it is even possible to receive energy via WISP and store it in the battery for future use.
All proposed techniques and protocols can be scaled up to support more than two radios. Important in this scenario is that each of the radios should have either a lower energy consumption or a longer range than the other radios.
Monitoring
Currently in the pilot phase, BLISP has numerous potential applications. It could prove extremely useful for agriculture, for example. Many cows carry sensors that share information with the base station in the cowshed. When the cows go into the fields, active radio is required in order to send data. BLISP then switches over automatically in order to conserve as much energy as possible, making the battery last significantly longer.
The new systems could also be used for medical applications. If a doctor wishes to use a wireless or portable device to monitor a patient’s ECG or EEG, BLISP makes this possible in the patient’s room using backscatter radio. If the patient goes to the toilet or the hospital canteen, for example, active radio then takes over contact. BLISP can be used to monitor not only people and animals. It can also be used to check the condition of goods fitted with this system.
Advantages
- Where possible, passive radio always takes precedence in order to conserve energy.
- The battery receives some charge while passive radio is in use.
- BLISP enables an optimum range at all times.
Highly effective drag reduction system
Highly effective drag reduction system
Due to the large length of some flat-bottomed boats, there is a large contact surface between the bottom of such vessels and the water. This causes a lot of drag during movement of the vessel in the water. A team of TU Delft scientists developed a solution to reduce this drag substantially.
Flat-bottomed vessels are primarily built for river and canal transport of heavy goods or persons. As some vessels are not self-propelled, they need to be towed or pushed by towboats. These towboats can easily tow or push up to six such vessels. As a result of the large contact surface of those vessels there is a lot of drag during transportation.

Drawbacks prior state of art
The TU Delft invention is based on the principle of ship drag reduction by air lubrication. Isolating a part of a hull from water by air reduces the wetted area, and thus the drag of a vessel. Many types of air lubrication have been suggested in the past, but in spite of many investigations and inventions, air lubrication has not been widely applied in practice on cargo vessels. The reason for this is that existing technologies have some major drawbacks. Prior state of art has a low efficiency in general and is too complicated to be applied and used. Moreover prior state of art is limited to specific ship types and not suitable to be used at all conditions.

Efficient design
The TU Delft invention solves existing technological problems by means of a simple and efficient design of the air cavities system. This air cavity system is a separate system that can be installed to the bottom of existing vessels. The air cavity system creates a number of cavities by injecting compressed air. Depending on the velocity of the vessel and the water depth a number of cavities in longitudinal direction can be selected to minimize the wetted area of the bottom. In order to guarantee the stability of a vessel with respect to roll motion it’s possible to select a number of cavities in the traversal direction.
Forward and backward
Besides controlling the number of cavities it is also possible to control the length of the cavities. Unique is the fact that the cavities can be formed when a vessel moves in forward as well as in backward direction. As installing this system does not require major modifications of a vessel, this invention is more costeffective to apply than prior state of art. As the system can be applied to existing and newly built vessels, this invention is very interesting for shipbuilding companies as well repairing companies and shipyards. Fuel savings in the order of 10 till 20% are realistic.
Advantages
- high efficiency at different velocities and water depths
- fuel savings 10 - 20%
- more cost-effective solution than prior state of art
- easy to manufacture, install and operate
- highly functional in both forward and backward direction
- can be applied to existing and newly built vessels
- suitable for inland waterway ships, barges, containerships and cruiseboats
Detection method for squats
Detection method for squats
Damage to the rails on railways often occurs as a result of the contact between the moving wheels and the rail surface, a phenomenon known as rolling contact fatigue, or RCF. Every year, the costs associated with RCF run to tens of millions of euros, and those are just direct maintenance and repair costs. Early detection of rail damage can not only save millions, but potentially lives as well.
Squats start out as minor rail top defects on straight sections or in slight bends of the tracks. They can arise from damage caused by train wheels sliding and spinning, or from rail corrugation. The wear or deformation of welds can also cause squats to develop. Every time a train passes over them, the squats become larger. In time a nick will grow into a crack and this can lead to rail breaks or even derailment.

The necessary repairs involve disruption and cancellation of trains, as well as high costs. Squats also represent a risk to safety. In the Netherlands, the entire rail network is subject to a visual inspection twice a year, sometimes with the help of photographs and video images. That takes a huge number of man-hours. Ultrasonic trains are also used to detect squats, but these are only capable of finding cracks deeper than seven millimetres. A new detection method measures the axle box accelerations at the wheel, making it possible to find squats that are still less than 0.2 mm deep.
Axle box accelerations are vibrations caused by the track or by the wheel set, or by rail top defects as the wheels move over them. The squat detection method registers both longitudinal and vertical axle box accelerations. From these, the signals caused by the track and/or the wheel set can be filtered out as these possess different frequency characteristics from signals caused by defects. Thus by analysing the vibrations measured at the axle box, the signal-of-interest, i.e. the defect, can be detected.
Advantages of squat detection
Railway companies can save millions on maintenance and repairs with this new squat detection method. The sooner defects are detected; the cheaper it is to fix them. And with train schedules getting ever busier, a preventive and predictive maintenance schedule can avoid emergency repairs. This will not only save money but will enhance the reputation of railways as a safe and reliable mode of transport.

Plant varieties
Wageningen University & Research licenses proprietary plant varieties of fruits and arable crops, ornamentals, mushrooms and woody crops developed in dedicated breeding programs.

Plant varieties
Wageningen University & Research licenses proprietary plant varieties of fruits and arable crops, ornamentals, mushrooms and woody crops developed in dedicated breeding programs.
Summary
Wageningen University & Research has a long standing tradition in developing new varieties in fruits, mushrooms, arable and woody crops and ornamentals. All varieties are either protected by plant breeders’ rights and/or plant patent in the US. Through extensive testing programs and market research, many of our varieties are being used by growers to reach satisfied consumers.
The varieties
Wageningen University & Research scientists have developed the following proprietary varieties:
- Apple: Bellida, Elise, Elshof, Elstar, Elstar Reinhardt, Fresco, PRI 1990-022-159, PRI 1990-045-133, PRI 1993-018-037, Red Elstar, Santana
- Pear: Verdi
- Strawberry: Elsanta, Lambada, Polka, Rapella
- Quinoa: Atlas, Carmen, Pasto, Riobamba
- Crambe: Galactica, Nebula
- Cannabis: Chamaeleon
- Iris: Casablanca, Deep River
- Lily: White American
- Calendula: Carola
- Oyster mushroom: Allerpo, Broncoh, Spoppo
- Perennials: Agastache ‘Heatwave’, Aster ageratoides ‘Starshine’, Crataegus succulenta ‘Jubilee’, Hypericum x inodorum ‘Arcadia’, Lonicera ‘Honey Baby’, Pieris japonica ‘Passion’, Rhododendron ‘Centennial Gold’, Rhododendron ‘Millennium Gold’, Skimmia japonica ‘Temptation’
Applications
The Wageningen University & Research breeding programs release since years outstanding varieties with good taste and shell-life, resistance against diseases and improved cultivation qualities. The varieties are available by licensing. For this we also collaborate with the company Fresh Forward, a spin-off of Wageningen University & Research that manages our licenses in fruits, arable crops and ornamentals, and with AbbottAgra, our partner for breeding and licensing of quinoa varieties.
Our scientists can assist breeders and growers interested in developing new varieties especially for bio-based or industrial applications. Our assistance may vary from technical assistance by performing special techniques, hiring greenhouse space, and performing commissioned, dedicated breeding programs.
Benefits
- Long standing breeding tradition and expertise
- Unique varieties with outstanding characteristics
- Embedment in excellent research environment
Stage of development
Introduction/implementation of variety. Ready for production and validated.
Measurement equipment hemispherical light transmission of transparent materials
Wageningen Plant Research Horticulture is seeking commercial partners interested in commercialising measurement equipment for determining the hemispherical light transmission of transparent materials such as structured glass, textiles, plastics.

Measurement equipment hemispherical light transmission of transparent materials
Wageningen Plant Research Horticulture is seeking commercial partners interested in commercialising measurement equipment for determining the hemispherical light transmission of transparent materials such as structured glass, textiles, plastics.
Summary
Greenhouse coverings, screens or solar panels are currently characterised by a measurement of the perpendicular light transmission of the materials. However, the perpendicular light transmission is not a relevant optical characteristic in order to determine the amount of available solar radiation or photosynthetic active radiation available for crops in greenhouses or protected structures.
Structured glass, inhomogeneous textiles such as screens and nettings, structured plastic panels, diffuse plastic films or coatings on glass require a representative measurement of optical properties on large samples due to structure size or inhomogeneity. For that purpose, a new method and measuring device has been developed to properly measure the required optical properties.
The invention
Wageningen University & Research scientists have developed a method and prototype equipment in order to measure the hemispherical light transmission of transparent materials such as structured glass, textiles, plastics, screens and nettings used in horticultural or agricultural application.
Applications

Commercially available equipment for quantifying the hemispherical light transmission of transparent materials is needed in order to get information on the amount of solar radiation and specifically the amount of photosynthetic active light entering through the specific transparent material. Transparent materials applied in horticulture or agriculture are for example greenhouse coverings, such as glass, plastic films, plastic sheets or textiles such as screens and nettings. The hemispherical light transmission of such materials correlates strongly with the average amount of light available in a greenhouse on crop level if the materials are applied. The equipment and measurement method is needed by material producers in order to control their production by measuring relevant optical characteristics and in order to develop new materials. Also the solar industry could benefit from the equipment since the same information is relevant for solar panels.
Benefits
- Correct quantification of hemispherical light transmission under different angles of light incidence by transparent materials.
- Accurate measurement of angular dependent transmission of structured glasses and inhomogeneous textiles.
- Measurement results strongly correlate with average light level in greenhouses available for crops (see also the publications mentioned under the section Additional information).
- Relevant for worldwide characterisation of transparent materials used as greenhouse coverings or screens in greenhouses.
- Relevant information for solar panels can be obtained.
Stage of development
Prototype phase – Method for measurement of hemispherical light transmission has been developed during the past years. Several prototypes of equipment have been build and tested. The current version has been validated and is used to perform measurements on a commercial basis for third parties. It therefore is suitable as the basis for further commercialisation. Existing clients have expressed their interest in purchasing a measurement device.
Pasta highly enriched with vegetables
Wageningen University is seeking commercial partners interested in producing and commercializing pasta with high vegetable content.

category_octrooi_patent
Pasta highly enriched with vegetables
Wageningen University is seeking commercial partners interested in producing and commercializing pasta with high vegetable content.
The invention
A method for preparing pasta with high amounts of vegetables (up to 20%) with a limited loss of nutrients during cooking. Taking 100 grams of this pasta provides the equivalent of 200 grams of vegetable, which is the required nutritional amount per day. The pasta retains good texture and taste.
Applications
Children like pasta, and to a much lesser extent vegetables, though the latter are also essential. The ideal would therefore be to have a vegetable that tastes like pasta. This is possible with the current invention. In particular the high amount of vegetable in the pasta is unique.
Benefits
Ready to eat food that is healthy and tasteful at the same time. The enriched pasta contributes to a healthy diet. It addresses an opportunity to provide children with a healthy choice being the tasty choice.
Stage of development
Ready for roll out.
Plants with modified vascular tissue
Wageningen University & Research is seeking commercial partners interested in applications for plants with modified vascular tissue.

Plants with modified vascular tissue
Wageningen University & Research is seeking commercial partners interested in applications for plants with modified vascular tissue.
Summary
The invention relates to the modification of gene expression in plants in order to manipulate the growth and/or structure of a plant through modification of the amount of vascular tissue and/or location of the vascular tissue. Modulation of the amount or location of vascular tissue in a plant can be used to produce plants or plant-derived products with altered mechanical properties to suit a variety of industrial applications, such as biofuel or paper production.
The invention
Scientists from Wageningen University have found that modulating the amount of TMO5/LHW dimer in Arabidopsis affects the amount of periclinal cell divisions of vascular cells, thereby modulating the size of the vascular bundle (both increasing or decreasing). Furthermore, it was found that expression of both members of this heterodimer is sufficient to trigger additional periclinal divisions (and therefore more cell files) in any cell type tested. Thus, this invention allows to modulate the size of the vascular bundle and any other tissue or organ type in a precise dose-dependent manner.
Applications
- Modulating the amount of the TMO5/LHW allows for a dose-dependent control of vascular bundle size with many putative applications (see benefits).
- Modulating the amount of TMO5/LHW ectopically using tissue specific promoters allows for enlargement specific organ or tissue types by increasing the amount of cell files. In Arabidopsis this appears to work in all tested plant tissues. Therefore, applications are numerous (see benefits).
Benefits
- Increased biomass of the plant for bio-fuel and paper production in woody species
- Modulating wood properties by increasing/decreasing the amount of cells, not the total organ size, resulting in eg. harder/softer wood species
- Increasing source to sink transport of photosynthates, sugars and metabolites in tuber forming species by increasing the vascular bundle size
- Modulated vascular bundle size could have an effect of for example water usage in restricting environments or improving plant strength
Stage of development
We have proof of concept in Arabidopsis thaliana and Physcomitrella Patens. Transformation into relevant crop species including Rice, Tomato and Poplar is in progress in collaboration with other research groups. Due to strict conservation of TMO5 and LHW in all vascular plants and the fact that the TMO5/LHW dimer functions in the non-vascular Physcomitrella, it is highly likely that a similar effect will be seen in crop species.
Bacterial soft rot resistance
Wageningen University is seeking commercial partners interested in developing plant varieties containing a novel gene that confers resistance to bacterial soft rot.

Bacterial soft rot resistance
Wageningen University is seeking commercial partners interested in developing plant varieties containing a novel gene that confers resistance to bacterial soft rot.
Summary
Pectobacterium carotovorum (formerly known as Erwinia carotovora) causes economically important plant diseases. Pectobacterium carotovorum is most renowned to cause soft rot and blackleg of potato. Blackleg and soft rot are economically important as they reduce potato yield and cause downgrading or rejection of potato seeds in certification processes. Moreover, Pectobacterium carotovorum is capable to cause disease in vegetables, ornamentals and many different tree species.
Several control strategies thus far have been studied, but with limited success. For potato no cultivars are available that show resistance to blackleg and soft rot disease caused by Pectobacterium carotovorum. Several wild potato species are known to have a high resistance and offspring of crosses between these wild species and commercial potato varieties have been obtained. This has not led to resistant potato lines so far.
The invention
Wageningen University scientists have identified and cloned a gene, known as LecRK-I.9, from Arabidopsis thaliana which shows high levels of resistance to Pectobacterium carotovorum. When expressed in potato, this gene confers bacterial soft rot resistance in the foliage of the plant.Applications
Development of genetically modified potatoes and other Solanaceous plants, such as tomato, pepper or eggplant, that are resistant to bacterial soft rot. As LecRK-I.9 is known also to improve resistance to Phytophthora infestans (late blight) in potato, it should be feasible to improve resistance to two major potato pathogens in one go.Benefits
- Provides a new gene for resistance to bacterial soft rot
- May reduce expenses associated with crop loss
- Can be used in combination with other resistance genes
- May increase crop yields
Stage of development
Development phase – laboratory and greenhouse tested. Potato plants were transformed with the open reading frame of the LecRK-I.9 gene. They successfully exhibited the soft rot resistant phenotype.Resistance against parasitic weeds
Wageningen University is seeking commercial partners interested in developing plant varieties having enhanced resistance to parasitic weeds and/or other methods to control them.

Resistance against parasitic weeds
Wageningen University is seeking commercial partners interested in developing plant varieties having enhanced resistance to parasitic weeds and/or other methods to control them.
Summary
Parasitic weeds cause enormous yield losses in agriculture. Broomrapes (Orobanche spp.) and witchweeds (Striga spp.) are serious pests in many countries and the main cause of yield reductions, extensive labour needs and other cultivation costs. The rapid deregistering of old herbicides even further increases the need for solutions. The seeds of parasitic weeds will only germinate after induction by a chemical signal exuded from the roots of their host. Many of these compounds have been isolated and identified from a number of different plant species and are collectively called the strigolactones.
The invention
Wageningen University scientists have found that the strigolactones germination stimulants are synthesized via the carotenoid pathway, which allows for the first time to devise methods for reducing or increasing the production of these germination stimulants. This finding is used to create crop species that do not induce germination of parasitic plant seeds anymore and therefore are resistant to parasitic plants.
Applications
Development of (genetically modified) plants having enhanced resistance to parasitic weeds. Other possible applications are the development of specific control measures against parasitic plants based on strigolactone overproducing trap and catch crops.
Benefits
- Provides new strategies to improve plant resistance against parasitic weeds
- May reduce expenses associated with crop loss
- Can be used in combination with other resistance genes
- May increase crop yields
Stage of development
Concept and application tested in tomato. Needs proof of concept in more crops and field conditions.Improving Crop Yield under Zinc Limiting Conditions
Wageningen University is seeking commercial partners interested in developing plant varieties containing novel genes that regulate the hyper accumulation of zinc. Applications include adaptation to zinc deficiency, phytoremediation and/or bio-fortification.

Improving Crop Yield under Zinc Limiting Conditions
Wageningen University is seeking commercial partners interested in developing plant varieties containing novel genes that regulate the hyper accumulation of zinc. Applications include adaptation to zinc deficiency, phytoremediation and/or bio-fortification.
Summary
Zinc is an essential micronutrient for all living organisms. The improvement of plant’s ability to mitigate zinc deficiency and so improve crop yield under zinc limiting conditions has thus far been hampered by insufficient knowledge on the mechanisms and regulation of the zinc homeostasis network in plants. Zinc deficiency afflicts up to 40% of the world’s human population, mainly in developing countries where people depend on cereal-rich diets for sustenance. Bio-fortification of crops with zinc, using plant breeding and other genetic technologies, can offer a sustainable solution to this global problem. The zinc deficiency response may promote the development of zinc deficiency tolerant crops and of metal hyperaccumulator plants for phytoremediation of contaminated soil or water.
The invention
Wageningen University scientists have identified two closely related members of the Arabidopsis thaliana basic-region leucine zipper (bZIP) transcription factor gene family, bZIP19 and bZIP23, that regulate the adaptation to low zinc supply. Homologues of the bZIP genes regulating zinc deficiency tolerance or hyper accumulation appear to be a general plant phenomenon as they were found conserved in various species such as rice, potato, tomato, poplar, Medicago and wheat.
Applications
Development of (genetically modified) plants with improved zinc deficiency tolerance, thus improving crop yield under zinc limiting conditions. Other possible applications are zinc bio-fortification to alleviate human nutrition problems and phytoremediation strategies to clean contaminated soils or water.
Benefits
- Provides new strategies to improve plant tolerance to low zinc availability
- May improve yield of plants grown under suboptimal zinc availability
- May facilitate bio-fortification through increased bioavailable zinc content in edible parts of plants
- May improve the phytoremediation properties of plants in soils polluted by zinc/cadmium
Stage of development
Concept and application tested in Arabidopsis thaliana. Needs proof of concept in other plants.
New method for the production of the compounds with anti-cancer activity, Arglabin and Parthenolide
Wageningen University is seeking commercial partners interested in producing the anti-cancer compounds Parthenolide, Hydroxy-Parthenolide and Arglabin through microbial production platforms and/or plants expressing their biosynthesis genes.

New method for the production of the compounds with anti-cancer activity, Arglabin and Parthenolide
Wageningen University is seeking commercial partners interested in producing the anti-cancer compounds Parthenolide, Hydroxy-Parthenolide and Arglabin through microbial production platforms and/or plants expressing their biosynthesis genes.
Summary
Parthenolide and Arglabin occurs naturally in plants, such as Tanacetum parthenium (Feverfew) and Artemisia glabella (smooth Wormwood) and show strong anti-cancer activity (Guzman et. al, Blood. 2005; 105(11): 4163–4169). Dimethylaminoparthenolide is currently in phase I clinical trials and Arglabin dimethylamino adduct is a registered antitumor substance in the Republic of Kazakhstan. The lack of water-solubility and bioavailability limits so far the potential of Parthenolide as a drug.
To solubilize Parthenolide and Arglabin, extra and intensive chemical steps are needed to develop dimethylaminoparthenolide or the Arglabin derivative (Arglabin dimethylamino adduct). The lack of knowledge on the last step in the biosynthesis pathway of Parthenolide and the largely unknown biosynthesis of Arglabin blocked up to now the potential to directly produce these compounds via biotechnological production platforms, based for example on micro-organisms.
The invention
Wageningen University scientists have identified from Feverfew and Wormwood plants the key genes in the biosynthesis of Parthenolide, water-soluble Parthenolide (Hydroxy-Parthenolide) and Arglabin. When the genes are expressed in yeast and in Nicotiana benthamiana plants, both systems are able to produce de novo Parthenolide, the water-soluble Hydroxy-Parthenolide and Arglabin.Applications
Development of genetically modified plants and/or microbial production platforms for contained, continuous and direct production of the potentially anti-cancer compounds Parthenolide - in its water-soluble form - and Arglabin.
Benefits
- Provides biosynthesis genes for the production of Parthenolide in its water-soluble form and Arglabin
- May simplify the production of these potential anti-cancer compounds
- May form the basis of a continuous, efficient production process
- Production can be optimised and improved to cut costs
Stage of development
Development phase – laboratory tested. Yeast and plants were transformed with the genes and they successfully produced the target compounds.Antiviral RNA compounds against Influenza viruses
Wageningen University is seeking commercial partners interested in developing a new class of antiviral drugs based on RNA compounds able to reduce viral replication levels and potentially applicable to all Influenza viruses, including new pandemic strains.

Antiviral RNA compounds against Influenza viruses
Wageningen University is seeking commercial partners interested in developing a new class of antiviral drugs based on RNA compounds able to reduce viral replication levels and potentially applicable to all Influenza viruses, including new pandemic strains.
Summary
Current options to reduce the impact of Influenza virus infections include vaccination and the use of antiviral drugs. Both therapeutics show limits. Vaccines cannot provide immunity against new strains of viruses and manufacturing capacity may be insufficient when coping with sudden high demands. Antiviral drugs have the advantage to be immediately applicable and thereby may help to contain an emerging pandemic virus at its emergence. However, the efficacy of the two currently available classes of antiviral drugs, neuraminidase inhibitors (e.g. Tamiflu) and ion channel blockers, is limited and dependent on a very early start of treatment. Furthermore, increasing numbers of cases are being reported on resistance against the application of neuraminidase inhibitors. The antiviral RNA compounds proposed in this invention act on a very conserved mechanism within Influenza viruses that is different from the currently available classes of emerging drugs, thus offering possibilities for new antiviral drug design and for solutions to the above described limitations.
The invention
Wageningen University’s scientists have identified RNA compounds that are preferentially being used during the initiation of the Influenza virus genome transcription. Once made dysfunctional, these RNA compounds can reduce viral genome transcription and replication levels and therefore the level of virus titers and the development of infection. Positive in vitro and ex vivo results to date suggest that this new RNA molecules will show greater efficacy against pandemic virus strains.
Applications
New antiviral drug design leading to transcriptional reduction of Influenza viral genomic RNA segments. Since the RNA compounds rely on a sequence which is conserved among all Influenza (A, B and C) viruses, the RNA compounds can be functional as antiviral drug against all Influenza viruses, including new, emerging pandemic strains.
Benefits
- Provides RNA compounds inhibiting Influenza virus transcription/replication in vitro and ex vivo
- May be functional as antiviral drug against all Influenza viruses, including new emerging pandemic strains
- RNA compounds can be applied in relative low dosage to still outcompete other RNA molecules
- May offer solutions to the disadvantages shown by vaccines and other antiviral drugs
Stage of development
Concept and application tested in vitro and ex vivo. Needs proof of concept through (pre-)clinical research.
Technology Offer Plant varieties
Wageningen University & Research licenses proprietary plant varieties of fruits and arable crops, ornamentals, mushrooms and woody crops developed in dedicated breeding programs.

Technology Offer
Plant varieties
Wageningen University & Research licenses proprietary plant varieties of fruits and arable crops, ornamentals, mushrooms and woody crops developed in dedicated breeding programs.
Wageningen University & Research has a long standing tradition in developing new varieties in fruits, mushrooms, arable and woody crops and ornamentals. All varieties are either protected by plant breeders’ rights and/or plant patents in the US. Through extensive testing programs and market research, many of our varieties are being used by growers to reach satisfied consumers.
The invention
Wageningen University & Research scientists have developed the following proprietary varieties:
- Apple: the most famous variety developed by Wageningen University & Research is Elstar. Currently the following varieties are commercialised by Fresh Forward: Bellida, Elise, Elshof, Red Elstar, Santana, Fresco (Wellant), SQ133 (Allurel), SQ159 (Natyra, Magic Star, Sprank) and WUR37 (Freya).
- Pear: Verdi (commercialised by Fresh Forward).
- Strawberry: Lambada, Polka, Rapella (commercialised by Fresh Forward).
- Quinoa: Atlas, Carmen, Pasto, Riobamba, Dutchess. WUR also owns Jessie, Rouge Marie and Bastille. The licenses are managed by RADICLE.
- Crambe: Galactica, Nebula.
- Cannabis: Chamaeleon.
- Calendula: Carola.
- Oyster mushroom: Allerpo, Broncoh, Spoppo, Desmé.
- Potato: Double Fun (commercialised by HZPC), Lily Rose and Purple Rain (commercialised by Plantera), Vitalia (commercialised by Stet).
- Perennials: Agastache ‘Heatwave’, Aster ageratoides ‘Starshine’, Crataegus succulenta ‘Jubilee’, Hypericum x inodorum ‘Arcadia’, Lonicera ‘Honey Baby’, Pieris japonica ‘Passion’, Rhododendron ‘Centennial Gold’, Rhododendron ‘Millennium Gold’, Skimmia japonica ‘Temptation’.
Applications
The Wageningen University & Research breeding programs release since years outstanding varieties with resistance against diseases, improved cultivation qualities and where appropriate good taste and shelf-life. Varieties are available by licensing. For this we collaborate with different companies. The company Fresh Forward, a spin-off of Wageningen University & Research, is responsible for all strawberry, apple and pear varieties. RADICLE and AbbottAgra are our partners for breeding and licensing of quinoa varieties.
Our scientists can assist breeders and growers interested in developing new varieties in a wide variety of crops but especially for bio-based or industrial applications. Our assistance may vary from technical assistance by performing special techniques, hiring greenhouse space, developing molecular markers for specific traits, and performing commissioned, dedicated breeding programs.
Benefits
- Long standing breeding tradition and expertise
- Unique varieties with outstanding characteristics
- Embedment in excellent research environment
Stage of development
Introduction/implementation of variety. Ready for production and validated.
Interested? Please contact:
Technology Offer Unique separation technology: tasty juice, with less sugar and acid
Experts at Wageningen Food & Biobased Research have developed an innovative separation technology enabling both sugar and acids to be removed simultaneously from juice (fresh and diluted puree) while still preserving the sugar-acid balance, aroma and flavour. Wageningen University & Research is offering interested parties the opportunity to apply this unique technology to their production process.

Technology Offer
Unique separation technology: tasty juice, with less sugar and acid
Experts at Wageningen Food & Biobased Research have developed an innovative separation technology enabling both sugar and acids to be removed simultaneously from juice (fresh and diluted puree) while still preserving the sugar-acid balance, aroma and flavour. Wageningen University & Research is offering interested parties the opportunity to apply this unique technology to their production process.
Summary
Juices contain high levels of natural sugars, so their consumption can lead to a high intake of calories and to dental decay. Membrane separation technology is currently used to lower those levels and it does so successfully, but this process also alters the balance of sugars and acids in the juice and removes some valuable flavour and aroma components.
The invention
Experts at Wageningen have developed a unique technology enabling both sugar and acids to be removed from fresh juices and diluted puree-based juices, while still preserving an appropriate sugar-acid balance, as well as the aromas and flavours. The technology works by passing the juice over a membrane that removes sugar and acid. After prefiltration, the liquid passes through a number of cylinders containing resin particles which selectively adsorb sugars and acids, preserving the aroma and flavour components.
Applications
The technology can be used by fruit and vegetable juice manufacturers to reduce the sugar, acid and calorie levels of their products.
Benefits
- Fruit and vegetable juices with less sugar, acids and kilocalories, while still preserving the flavour
- Improved healthy image for the product
- Add-on technology: easy to build into existing production line
Stage of development
Prototype tested in simulated operational environment.
Technology Offer Improving Crop Yield under Zinc Limiting Conditions
Wageningen University & Research is seeking commercial partners interested in developing plant varieties containing novel genes that regulate the hyper accumulation of zinc. Applications include adaptation to zinc deficiency, phytoremediation and/or bio-fortification.

Technology Offer
Improving Crop Yield under Zinc Limiting Conditions
Wageningen University & Research is seeking commercial partners interested in developing plant varieties containing novel genes that regulate the hyper accumulation of zinc. Applications include adaptation to zinc deficiency, phytoremediation and/or bio-fortification.
Summary
Zinc is an essential micronutrient for all living organisms. The improvement of plant’s ability to mitigate zinc deficiency and so improve crop yield under zinc limiting conditions has thus far been hampered by insufficient knowledge on the mechanisms and regulation of the zinc homeostasis network in plants. Zinc deficiency afflicts up to 40% of the world’s human population, mainly in developing countries where people depend on cereal-rich diets for sustenance. Bio-fortification of crops with zinc, using plant breeding and other genetic technologies, can offer a sustainable solution to this global problem. The zinc deficiency response may promote the development of zinc deficiency tolerant crops and of metal hyperaccumulator plants for phytoremediation of contaminated soil or water.
The invention
Scientists of Wageningen University & Research have identified two closely related members of the Arabidopsis thaliana basic-region leucine zipper (bZIP) transcription factor gene family, bZIP19 and bZIP23, that regulate the adaptation to low zinc supply. Homologues of the bZIP genes regulating zinc deficiency tolerance or hyper accumulation appear to be a general plant phenomenon as they were found conserved in various species such as rice, potato, tomato, poplar, Medicago and wheat.
Applications
Development of (genetically modified) plants with improved zinc deficiency tolerance, thus improving crop yield under zinc limiting conditions. Other possible applications are zinc bio-fortification to alleviate human nutrition problems and phytoremediation strategies to clean contaminated soils or water.
Benefits
- Provides new strategies to improve plant tolerance to low zinc availability
- May improve yield of plants grown under suboptimal zinc availability
- May facilitate bio-fortification through increased bioavailable zinc content in edible parts of plants
- May improve the phytoremediation properties of plants in soils polluted by zinc/cadmium
Stage of development
Concept and application tested in Arabidopsis thaliana. Needs proof of concept in other plants.
Interested? Please contact:
Technology Offer Thermostable Cas9 Nucleases
The ultimate aim of plant breeding has always been to make plants resistant to drought and diseases. That could help eliminate hunger around the world. This is no longer a distant thought, thanks to a technology called CRISPR-Cas. Today Wageningen University & Research (WUR) announces it will provide potential partners with free licenses to work on its patented CRISPR technology. The license must be applied to gene-editing of plants for non-profit applications. “We hope this contributes to healthier, more sustainable, equitable, affordable and resilient food production for all”, says WUR President prof. dr. ir. Louise O. Fresco.

Technology Offer
Thermostable Cas9 Nucleases
The ultimate aim of plant breeding has always been to make plants resistant to drought and diseases. That could help eliminate hunger around the world. This is no longer a distant thought, thanks to a technology called CRISPR-Cas. Today Wageningen University & Research (WUR) announces it will provide potential partners with free licenses to work on its patented CRISPR technology. The license must be applied to gene-editing of plants for non-profit applications. “We hope this contributes to healthier, more sustainable, equitable, affordable and resilient food production for all”, says WUR President prof. dr. ir. Louise O. Fresco.
CRISPR-Cas is a technology that enables genetic material to be changed relatively simply, very accurately and efficiently. Worldwide there are over 3.000 CRISPR-Cas related patents, of which WUR also holds several. For five of them (which are jointly owned with Dutch Research Council NWO), WUR decided to provide free licenses. An article in Nature was published right after the announcement.
Why this is unique
Prof. Fresco: “This is really quite unique for CRISPR, in the academic world and beyond. As far as we know, we’re among the first to do so regarding CRISPR-technology. We do it, because we simply and firmly believe this is the right thing to do.
Two billion people face inadequate nutrition around the world in 2020. Nearly all of them are also vulnerable to the effects of climate change. So we need a transformation to healthier, more sustainable, equitable, affordable and resilient food systems. This will also take center stage during the UN Food Systems Summit on September 23rd. CRISPR and other biosciences could accelerate this transformation.
It therefore fits perfectly with the WUR mission: to explore the potential of nature to improve the quality of life. CRISPR, a bacterial defense system to viruses is a great example. A WUR team, led by prof. Van der Oost has been studying it since 2006.”

Potential of CRISPR-Cas in the fight against hunger
Microbiologist prof. dr. John van der Oost is a world leading authority on CRISPR-Cas. He is often described as one of the founding fathers of the technology and he took the initiative to provide free CRISPR licenses. He says: “The potential of CRISPR-Cas cannot be overstated. It is a very versatile technology that could provide new and sustainable ways to feed earth’s growing population. We happily share our knowledge to that end, and hope more patent holders will consider doing the same.”
Dr Mohamed H.A. Hassan, President of the Academy of Science for the Developing World (TWAS) and Chairman of the Governing Council of the United Nations Technology Bank for the Least Developed Countries welcomes the move by Wageningen University & Research: “Providing these free licenses potentially offers immediate solutions to a number of urgent problems in the world: growing food needs and the impact of both climate change and pathogens. I expect non-profit parties, agricultural and food-research institutes in low-income countries to benefit. These institutes are crucial for developing improved food and feed crops and food technology for local farmers and poor consumers. Without this initiative of WUR, they probably would not be able to acquire these licenses.”

Scientific collaboration around CRISPR
“The full potential of this technology can only be achieved through long-term partnerships and capacity building”, says prof. Fresco. “We would like to learn from our future CRISPR partners as well, and build upon their knowledge in return. Together, we could change the way we deal with food security around the world. This is why we are confident about giving away this knowledge, in the spirit of the Open Science movement: to make publicly available what has been funded with public money.
Marcel Levi, Chair of the Dutch Research Council (NWO): “It is fantastic to see where research can lead us. NWO has been involved since 2009 in the development of this revolutionary application of biotechnology, and as a co-patent holder this also makes us a little bit proud today. As a strong supporter of Open Science, we are very pleased that the licenses are being made available to contribute to a better world. This is an example of knowledge that has been developed with public money benefitting society. Science is helping to contribute to solving one of the great issues of our time.”
Serendipity
The announcement was made during the WUR opening ceremony of the academic year (OAY), with ‘Crossing Boundaries’ being this year’s theme. In interviews leading up to the OAY prof. Fresco urged for more serendipity and failure in scientific work: “I envision us bringing together groups of promising scientists without a direct research assignment. The most unorthodox ideas would then be allowed, along with masterful failures. Chance discoveries - of which CRISPR-Cas technology is an example - only come to light when you see them. We need to have the courage to look at reality in different ways.”
Interesse? Neem contact met ons op:
Technology Offer ThermoCas9: Stable and efficient gene editing, even at high temperatures
CRISPR-Cas is a microbial adaptive immune system that uses RNA-guided nucleases to cleave foreign genetic elements. CRISPR-based genome editing tools have revolutionised fundamental research and biotechnological applications in both eukaryotes and prokaryotes due to their speed, cost-effectiveness, and precision.

Technology Offer
ThermoCas9: Stable and efficient gene editing, even at high temperatures
CRISPR-Cas is a microbial adaptive immune system that uses RNA-guided nucleases to cleave foreign genetic elements. CRISPR-based genome editing tools have revolutionised fundamental research and biotechnological applications in both eukaryotes and prokaryotes due to their speed, cost-effectiveness, and precision.
New CRISPR applications for humans, plants, animals, fungi and microbes
ThermoCas9 is a novel CRISPR-Cas9 enzyme derived from a heat-loving bacterium. Unlike the commonly used SpCas9, ThermoCas9 functions across a broad temperature range (20–65°C), making it a versatile and robust genome editing tool for use in harsh or variable conditions.
What makes ThermoCas9 unique?
- Maintains stability and activity at elevated temperatures.
- Offers high precision and stricter target matching at lower temperatures.
- New variants show improved performance at 20–37 °C — ideal for plant and human applications.
- Strong cytosine base editing activity in human cells with exceptionally wide editing window
Potential applications
- Targeted genome editing for treating human blood or metabolic disorders.
- Breeding of stress-tolerant or high-yielding crop varieties.
- Genetic engineering of microbes and fungi
- Fundamental gene function studies in plants, animals, and humans.
- Tools for nucleic acid detection and disease marker identification.
Development stage & collaboration opportunities
The test is currently between TRL 3 and 4 (proof of concept) and ready for further development. Wageningen University & Research offers access to cutting-edge tools and expertise to support further development and application.
Interested? Get in touch with:
Technology Offer SHIKIMAX: biotechnological production of aromatics
The chemical industry faces increasing pressure to adopt more sustainable practices to reduce its environmental footprint. Microbial production of key chemical building blocks, such as aromatics, can offer a biobased alternative. Microbes have a natural pathway of aromatic production: the Shikimate pathway. However, this pathway is highly regulated, limiting the amount of carbon directed to aromatic production, making it difficult to achieve economically viable yields.

Technology Offer
SHIKIMAX: biotechnological production of aromatics
The chemical industry faces increasing pressure to adopt more sustainable practices to reduce its environmental footprint. Microbial production of key chemical building blocks, such as aromatics, can offer a biobased alternative. Microbes have a natural pathway of aromatic production: the Shikimate pathway. However, this pathway is highly regulated, limiting the amount of carbon directed to aromatic production, making it difficult to achieve economically viable yields.
At WUR, we have developed SHIKIMAX: a platform method to use this natural pathway to produce these aromatics with biotechnology. Specifically, a bacterial strain was developed that uses the the Shikimate pathway as its primary catabolic route. This was found to be very effective, resulting in the highest yields ever reported for the Shikimate pathway.

What makes our platform technology unique?
- The strain requires only glycerol as a carbon source, eliminating the need of expensive supplemental amino acid sources in the growth medium
- High yields; for one aromatic target, yields were reported of up to 81.2% carbon yield, which is 2.3x higher than that of the next best reported bacterial strain.
- Can be produced efficiently through a biotechnological process
- Patented technology
High-potential applications
Potential aromatics that can (potentially) be produced include 3-hydroxybenzoate, 4-hydroxybenzoate, salicylate, anthranilate and para-aminobenzoate, which can all function as precursors to other aromatics. Applications of these aromatics and their products include:
- Aspirin production from salicylate
- Fragrances (e.g. with vanillin or anisole)
- Food additives (e.g. 4-hydroxybenzoate, anthranilate, vanillin)
And other applications can be found across agrochemicals, pharmaceuticals, dyes, polymers, cosmetics, adhesives and antimicrobial products.
Development stage & collaboration opportunities
The technology is at TRL 3-4 and a patent application has been filed. The effectiveness of SHIKIMAX has been demonstrated in the laboratory with the industrial bacterial host Psuedomonas putida (P. Putida) for the production of salicylate, 4-hydroxybenzoate and 3-hydroxybenzoate, all of which are key aromatic precursor molecules for various industrial applications. Further productivity optimalisation is in progress. Wageningen University & Research offers technical expertise to support further development and application.
Interested? Get in touch with:
Technology Offer Making biodegradable plastics from syngas: a biotech solution to waste and plastic pollution
Syngas is a mixture of CO, H2 and CO2 that can be produced from the gasification of renewable feedstocks, such as biomass, organic waste, or industrial residues. Chemical conversion of bio-based syngas is challenging due to the presence of impurities like sulfur compounds. However, biological conversion is well-suited to bio-based syngas, because some microbes can tolerate well – and sometimes even benefit from – these impurities. A novel fermentation process was developed to produce biodegradable polyester plastics using syngas (or other CO-rich streams) as a feedstock.

Technology Offer
Making biodegradable plastics from syngas: a biotech solution to waste and plastic pollution
Syngas is a mixture of CO, H2 and CO2 that can be produced from the gasification of renewable feedstocks, such as biomass, organic waste, or industrial residues. Chemical conversion of bio-based syngas is challenging due to the presence of impurities like sulfur compounds. However, biological conversion is well-suited to bio-based syngas, because some microbes can tolerate well – and sometimes even benefit from – these impurities. A novel fermentation process was developed to produce biodegradable polyester plastics using syngas (or other CO-rich streams) as a feedstock.
Microbial conversion of CO-rich gases is already applied at scale, typically using pure cultures of acetogens (e.g. Clostridium autoethanogenum), to produce simple molecules like ethanol and acetate. Our innovation lies in the use of microbial mixed cultures to establish a microbial food-web, enabling the production of more complex molecules. A simplified example is given in the figure below. Microbe A can produce polyhydroxyalkanoates (PHA), a biodegradable plastic. It uses CO for energy, releasing H2 and CO2 as by-products. Microbe B then converts these gases into acetate – an essential carbon source for Microbe A to produce PHA. Together, the two microbes transform CO into bioplastic in a single, integrated fermentation process.

What makes our system unique?
- Bioplastic production from CO-rich syngas, compatible with syngas derived from renewable or waste feedstocks
- Production of C4-C5 PHA blends for customisable material properties
- Integrated defined co-culture or (enriched) mixed culture in a single reactor, simplifying operation and increasing efficiency
- No need for added sugars or organic acids – PHA is produced directly from the gaseous substrates alone
- Patented technology
High-potential applications
- Valorisation of syngas generated from renewable feedstocks (biomass, wastes), with minimal gas purification required
- Carbon capture and conversion via microbial fermentation
- Sustainable PHA production, including C4-C5 PHA blends
- Carbon-neutral or carbon-negative bioplastic manufacturing
Development stage & collaboration opportunities
The technology is at TRL 4 and protected by IP. Several commercial parties have shown interest. Wageningen University & Research offers technical expertise to support further development and application.
Interested? Get in touch with:
Technology Offer IceBinders: artificial antifreeze proteins
Some organisms have the capacity to survive at sub-zero temperatures thanks to unique proteins that selectively bind to ice crystals. Wageningen University and Eindhoven University of Technology have collaboratively designed artificial versions of such ice-binding proteins that can prevent the growth of large ice crystals. Together, WUR and TU/e are looking for partners to realize valuable applications for this new technology.

Technology Offer
IceBinders: artificial antifreeze proteins
Some organisms have the capacity to survive at sub-zero temperatures thanks to unique proteins that selectively bind to ice crystals. Wageningen University and Eindhoven University of Technology have collaboratively designed artificial versions of such ice-binding proteins that can prevent the growth of large ice crystals. Together, WUR and TU/e are looking for partners to realize valuable applications for this new technology.
Upon freezing and thawing, ice crystals can cause a lot of cellular damage to biological tissues. It has been proven in proof-of-concept studies that ice-binding proteins can protect cells against some forms of freezing damage. The designed ice-binding proteins are thermostable and can be produced in bioreactors using suitable host microorganisms such as E. coli.
What makes our system unique?
- Computationally designed artificial ice-binding proteins
- High thermal stability and high activity
- Can be produced efficiently through a biotechnological process
- Patented technology
High-potential applications
- Organ and tissue transport and preservation
- Preservation of live cells (e.g. cell cultures for research, CarT cells, cells for IVF)
- Preventing freeze damage in frozen food
Development stage & collaboration opportunities
The technology is at TRL3/TRL 4 and protected by IP. The proteins have been produced at lab scale. Proof-of-concept cryopreservation studies have been performed using live cells. Wageningen University & Research and TU/e are actively taking steps to work on further product development.
Interested? Get in touch with:
Technology Offer Trapping plant pests: a physical alternative to chemical pesticides
Chemical pesticides pose a huge threat to ecosystems, since they reduce biodiversity and disrupt essential ecological processes. They have also been linked to serious human health risks. With regulatory and societal pressure increasing, there is a large need to reduce the use of chemical pesticides in crop cultivation without a loss in production. The group of dr. Thomas Kodger, respected WUR scientist with over 70 published articles to his name, has developed a biobased alternative to chemical pesticides: a sprayable sticky liquid that can trap pests on the plant surface.

Technology Offer
Trapping plant pests: a physical alternative to chemical pesticides
Chemical pesticides pose a huge threat to ecosystems, since they reduce biodiversity and disrupt essential ecological processes. They have also been linked to serious human health risks. With regulatory and societal pressure increasing, there is a large need to reduce the use of chemical pesticides in crop cultivation without a loss in production. The group of dr. Thomas Kodger, respected WUR scientist with over 70 published articles to his name, has developed a biobased alternative to chemical pesticides: a sprayable sticky liquid that can trap pests on the plant surface.
A new method was developed for making a sprayable liquid suspension with sticky oil-based particles in water. The oils used are vegetable oils, which makes this a biological method of protecting plants from pests. By only treating the oils with oxygen while heating and then cooling the oils in a controlled way, the perfect properties are achieved. This oil is then ground into particles and placed in water for spraying. The spray was found to be effective for capturing thrips, a common plant pest. This greatly reduced the damage caused by thrips to the crop.
What makes our product unique?
- Non-toxic
- From a biological source (biobased)
- Alternative to chemical pesticides
- Patented technology
High-potential applications
The technology can be used for protecting virtually any type of plant, including:
- Ornamental horticulture
- Horticulture edibles
- Arable crops
Development stage & collaboration opportunities
The technology is at TRL 4 and protected by IP. The sprayable suspension has been developed at a laboratory scale and has been tested on live Chrysanthemum plants for its ability to capture thrips both adult and larvae. Additional testing on tomato plants, including testing for the efficacy against additional pests, is under development. A parallel project has been launched to investigate the biodegradability of the spray in a natural environment.
WUR is looking for partners that are interested in licensing this technology, investors for further product development and (field) research collaborators.
Interested? Get in touch with:
Cardiac coronary artery bypass implant
Julius Research Toolbox
IL4-IL10 Synerkine for treating OA
The present invention is in the field of immunology and pharmacology, particularly for treatment of osteoarthritis, chronic pain, inflammatory diseases or disorders, and related diseases and disorders. The invention particularly relates to a novel fusion protein comprising interleukin 4 (IL-4) and interleukin 10 (IL-10), optionally physically fused together by a linker. Particularly, the present invention provides an IL4-IL10 fusion protein endowed with a superior biological activity over a combination of the individual cytokines. The present invention also provides nucleic acid sequences encoding a IL4-IL10 fusion protein, expression vectors comprising such nucleic acid sequences, host cells or host organisms altered to harbour the nucleic acid sequence encoding the IL4-IL10 fusion protein and the IL4-IL10 fusion protein itself. The invention further provides methods for producing an IL4- IL10 fusion protein using a cell or organism harbouring such nucleic acid sequences.
Transgenic organisms comprising the nucleic acid sequence of the invention are also provided. The present invention also relates to pharmaceutical compositions comprising the IL4-IL10 fusion protein. Finally, the use of the IL4-IL10 fusion protein as a medicament, in particular for the prevention and/or treatment of osteoarthritis and of conditions characterized by local or systemic inflammation, immune activation, lymphoproliferation and/or chronic pain is taught herein.
Inventors
van Roon J.A.G., Hartging S.A.Y., Hack C.E., Louws C., Lafeber F.P.J.G.
Applicant
UMC Utrecht Holding B.V.
Patent Status
PCT application filed: WO2013070076
Liaison
Bas Nagelkerken
bas.nagelkerken@utrechtholdings.nl
030 253 3621
Novel CD20 mAbs
D20 is a notoriously difficult antigen to raise immune responses to and hence only few high affinity monoclonal antibodies exist, amongst which the clinically used rituximab (Rituxan) and ofatumumab (Arzerra). In search for even better monoclonal antibodies the research group of Dr. J.H.W. Leusen of the Immunotherapy group at the UMC Utrecht used a novel immunization protocol, which yielded surprisingly high numbers of responders, never seen before. Extensive characterization of the newly obtained hybridomas showed that the panel of mAbs contain unique types of high affinity antibodies, that incorporate all required effector functions needed for clinical efficacy.
The panel was tested for apoptosis induction of tumor cells, complement activation and antibody dependent cellular cytotoxicity (ADCC) and showed high efficacies, some antibodies even better than clinical CD20 antibodies. Sequences and epitope mapping is available.
Isolated antibodies or Hybridomas are available for licensing.
Inventors
Leusen J.H.W.
Applicant
UMC Utrecht Holding B.V.
Liaison
Bas Nagelkerken
bas.nagelkerken@utrechtholdings.nl
030 253 3621

