



Concord: Lifesaving care for preterm babies improved by keeping the umbilical cord intact neonatal care, incubator, cord clamping
neonatal care, incubator, cord clamping
Concord: Lifesaving care for preterm babies improved by keeping the umbilical cord intact

Background:
Every year, 15 million infants are born preterm worldwide. Preterm birth is responsible for over 1 million deaths each year due to complications at birth, many survivors suffer from long-term disability, including learning problems, cerebral palsy or chronic lung problems.
Most preterm infants breathe insufficiently at birth, the cord is clamped immediately to not delay the respiratory support they need to survive. However, immediate cord clamping compromises the infants’ cardiovascular function, which can injure its immature organs. Waiting with cord clamping until the infant has been stabilized potentially reduces complications at birth, long term disabilities and mortality.
Technology Overview:
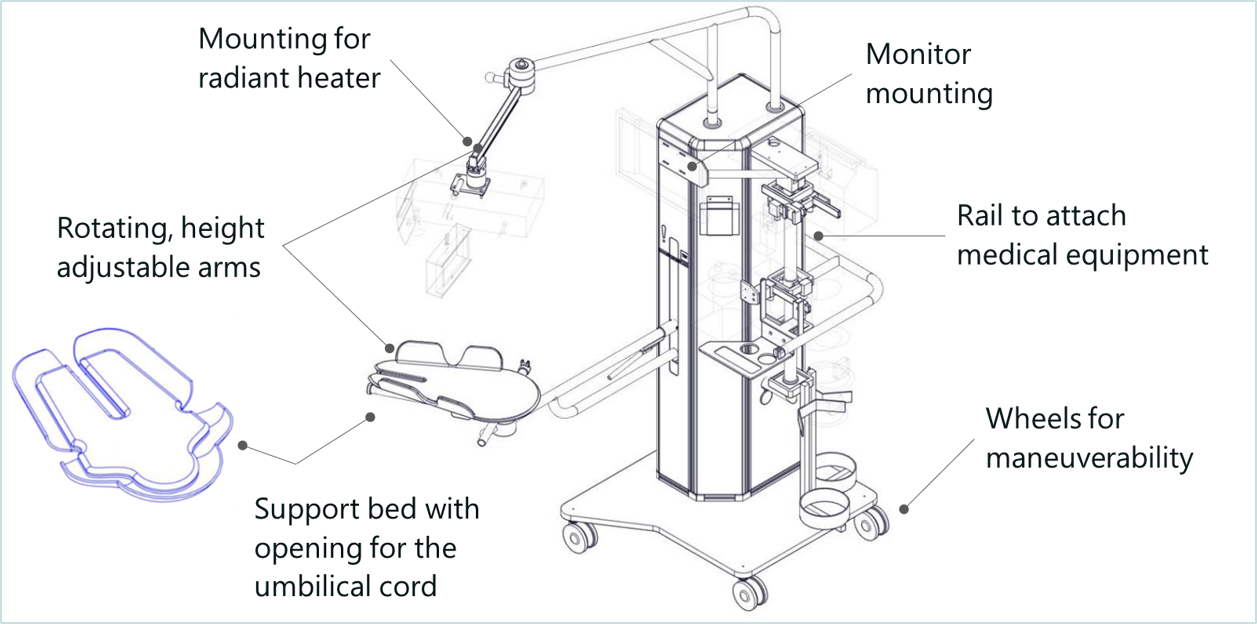
With Concord, delayed cord clamping for preterm babies requiring lifesaving care will now be a safe option. Concord is an innovative resuscitation table that makes it possible for the neonatologist to provide all care needed to stabilize the baby, while the umbilical cord remains intact. Concord has an adjustable support bed that can be positioned closely above the mother, on which the baby can be placed safely immediately after birth, to keep the sometimes very short umbilical cord intact. In addition, Concord keeps the baby close to the mother to allow bonding.
Benefits:
Many cord clamping studies compared immediate cord clamping with delayed cord clamping, focusing on breathing infants:
• Fewer babies needed blood transfusions for anemia – Relative Risk (RR) 0.61
• Reduced risk of bleeding in the brain (IVH) – RR 0.6
• Reduced risk of necrotizing enterocolitis (NEC) – RR 0.62
There is now data available showing another large benefit in delaying cord clamping until ventilation has been established. Results show that waiting with cord clamping until the lung has aerated and the infant has been stabilized leads to placental transfusion and a more stable oxygenation of the blood and a more stable heart rate during transition. This potentially decreases the injury to the infants immature organs, especially the brains and intestines. Concord therefore has the potential to:
• Reduce infant mortality;
• Reduce acute complications at birth;
• Reduce medical interventions;
• Less days in neonatal care units;
• Reduce the risk of long term disability;
• Reduce the societal economic cost of preterm birth.
Further Details:
Concord Neonatal B.V. was founded in April 2017 as a spin-out from LUMC, for the development and global commercialization of Concord.
Potential Applications:
Concord offers a solution for all worldwide hospitals specializing in the care of preterm newborns, to improve childbirth care for all newborns requiring resuscitation.
State of Development:
Specialists at Leiden University Medical Center (LUMC) invented Concord and developed a clinical prototype. The prototype of Concord is used for validation of feasibility and safety in a Phase 1 clinical study. Today, 28 babies have been successfully delivered using Concord at LUMC, in the delivery room as well as in the operating room. The results are very promising regarding the feasibility and safety of the workflow, the quality of care for the baby and the very positive feedback from parents.
Concord Neonatal aims to launch a commercial product by the end of 2018. To achieve this goal, Concord Neonatal is looking for €750,000 in external investment in 3 tranches of €250,000 in 2018, 2019 and 2021.
Liposome drug delivery vector targeting the blood brain barrier pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
Liposome drug delivery vector targeting the blood brain barrier
Technology Overview:
Researchers at Leiden University have developed a novel lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle with a selectivity for the brain endothelium (the blood brain barrier or BBB) of >10-fold over the systemic endothelium. This means that not <1% percent of the injected dose gets delivered to the brain and/or BBB, as is now the case with doxorubicin-filled liposomes, but potentially a 10-fold selectivity for brain endothelium over systemic endothelium or more of a drug can be delivered directly to the brain and/or BBB.
Potential Applications:
Drugs specifically targeting the brain and/or the BBB, such as treatments for strokes, cancer and neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, Huntington’s).Enhancement of brain and/or BBB (theranostic) imaging.
State of Development:
The BBB-selectivity was shown in zebrafish, that have a genome which is 70% homologous to humans and show a very similar brain morphology, organization and expression of key markers for BBB-function and integrity. Experiments in mammalian models are currently being undertaken.
The researchers have also demonstrated proof-of-principle of successful encapsulation of small molecule drugs as well as larger cargoes in the newly developed nanocarrier.
Mouse models of spontaneous thrombosis animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
Mouse models of spontaneous thrombosis
Background:
Thrombosis comes in two flavours:
- VT: Venous thrombosis (with pulmonary embolism as possible results)
- AT: Arterial thrombosis (with myocardial infarction or stroke as possible result)
Venous and arterial thrombosis are a major source of morbidity and mortality worldwide and both are complex vascular diseases for which pathogenesis is incompletely understood. Animal models are fundamental in our effort to understand the disease and develop better therapy (“Holy Grail” an antithrombotic without bleeding risk as side-effect).
Currently there are limited (venous thrombosis) or no (arterial thrombosis) technically reproducible or clinically relevant mouse models for these diseases.
Technology Overview:
Researchers at the LUMC have developed a mouse model for VT via “humanizing” mouse coagulation via RNAi of the hepatic antithrombin (Serpinc1) and protein C (Proc) genes.
In addition, they have developed a mouse model for AT via RNAi of Proc in Apoe-/- mice. In initial studies organized and large thrombi superimposed on an aortic root atherosclerotic plaque were observed, a unique and novel finding. This model needs further optimization.
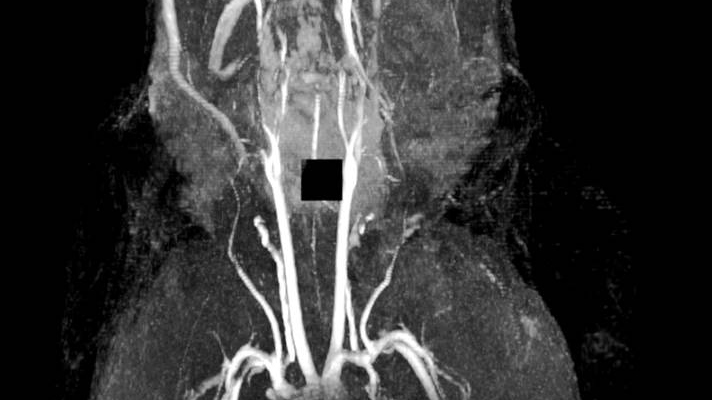
Figure 1: MRI of spontaneous venous thrombosis in a large vessel in the head (mandibular area)
Benefits:
The VTE model:
- Generates highly reproducible acute venous thrombosis
- Is technically simple and fast
- Reproduces morphologically venous thrombosis in humans
- Is responsive to (pharmacological) thrombin and platelet inhibition
- Is used by LUMC researchers and others to study VT pathogenesis
The AT model:
- Is technically simple and fast (i.e. the thrombosis part)
- Is reproducible, but has a low incidence (0.16)
- Responsiveness to drugs currently used to prevent AT unknown
- The occurrence of spontaneous atherothrombosis in the siProc apoE-/- mice is a truly unique event so far lacking in other preclinical models.
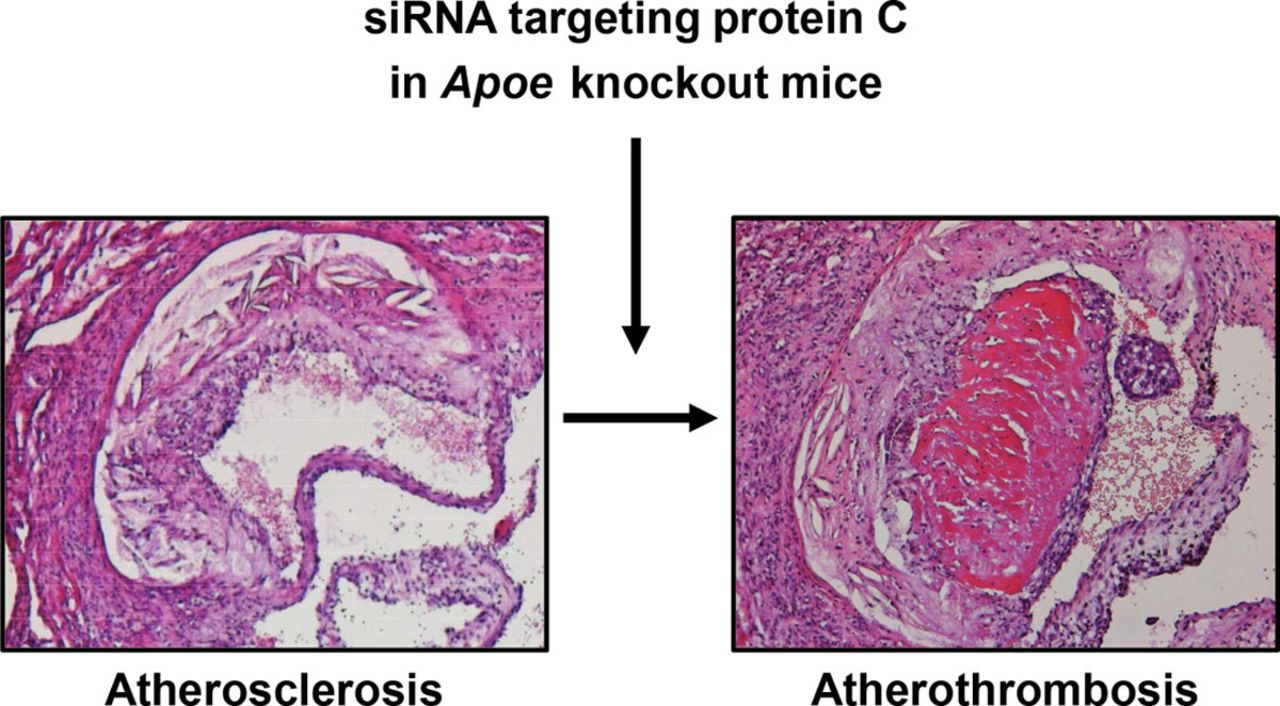
[Figure 2: Arterial (athero)thrombosis in apoliporotein E deficient mouse following RNAi]
Further Details:
VTE model: Heestermans et al., Blood. 2016 May 26;127(21):2630-7
AT model: Ouweneel et al., Arterioscler Thromb Vasc Biol. 2017 May;37(5):782-785
Applications:
Potential applications would be in preclinical research of venous thrombosis and pulmonary embolism, and arterial thrombosis and myocardial infarction and stroke.
Venous Thrombosis and Pulmonary Embolism (source: World Thrombosis Day 2016)
- Every year, there are approximately 10 million cases of VTE worldwide
- In the U.S., there are 100,000 - 300,000 VTE-related deaths every year
- In Europe, there are 544,000 VTE-related deaths every year
- Up to 60 percent of VTE cases occur during or after hospitalization, making it a leading preventable cause of hospital death.
Arterial Thrombosis and Myocardial Infarction and Stroke (source Netherlands Heart Foundation)
- Every day over 100 related deaths in The Netherlands
- Every day over 1000 cases hospitalized in The NetherlandsOver 1 million cases in The Netherlands
Opportunity:
Know-how on the VTE model is available for partnering and/or licensing.
LUMC are seeking co-development partners to further optimize the AT model and/or study its response to drugs that are currently used in MI/stroke (lipid-lowering/antiplatelet drugs) and to those in current pipelines (PAR inhibitors, FXI inhibitors, others).
Plasma biomarker for detection of onset of chronic Rheumatoid Arthritis Rheumatoid Arthritis, biomarker, plasma
Rheumatoid Arthritis, biomarker, plasma
Plasma biomarker for detection of onset of chronic Rheumatoid Arthritis
Background:
Rheumatoid Arthritis is characterized by inflammation of joints resulting in joint damage and disability. Research demonstrated that the presence of Rheumatoid Arthritis specific auto-antibodies directed against citrullinated proteins (ACPA) in the serum of patients increased the risk of developing the disease in patients with pain in their joints. ACPA can already be detected in patients years before onset of disease.
Technology Overview:
Researchers at the LUMC have recently discovered that ACPA isolated from Rheumatoid Arthritis patients is decorated with unique sugar structures. These sugar structures are not, or to a lesser extent, present on ACPA of people not yet diagnosed with Rheumatoid Arthritis. This unique sugar structure attached to ACPA could represent a biomarker of the transition phase from healthy to disease. Currently, researchers at the LUMC are investigating the possibility of a diagnostic test to analyse the sugar structures attached to ACPA on a large scale. Based on this test, the sugar structures of ACPA can predict the development of Rheumatoid Arthritis in patients with joint pain. This knowledge is important for clinicians to select an appropriate treatment in time to prevent progression towards chronic Rheumatoid Arthritis.
%2Bfigure%2B1.jpg) [Figure 1: ACPA with unique sugar structures are specifically present in patients with Rheumatoid Arthritis and might be crucial to predict progression from auto-antibody positive healthy subjects to patients with full-blown, chronic and persistent arthritis.]
[Figure 1: ACPA with unique sugar structures are specifically present in patients with Rheumatoid Arthritis and might be crucial to predict progression from auto-antibody positive healthy subjects to patients with full-blown, chronic and persistent arthritis.]
Benefits:
ACPA appear in the blood of Rheumatoid Arthritis patients up to ten years before onset of disease. Currently it is impossible to predict the exact time point of disease manifestation. Therefore, treatment starts at diagnosis of the disease. At this stage, the disease is already chronic, and the patient is devoted to lifelong treatment. This invention allows clinicians to intersect the healthy auto-reactive positive pre-disease phase from the pathogenic phase in which Rheumatoid Arthritis development commenced. If in this stage therapy is applied, development towards Rheumatoid Arthritis might be stopped before chronification occurs.
Further Details:
More background information can be found in the publication: “Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis.”, Rombouts et al., Ann Rheum Dis. 2016 Mar.
Potential Applications:
Identify ACPA+ individuals with high risk to develop Rheumatoid ArthritisTargeted treatment - distinguish individuals with high risk to develop Rheumatoid Arthritis in the “at risk” group and start treatmentEarlier start of treatment to prevent/delay/decrease severity and chronification of Rheumatoid Arthritis
Opportunity:
The researchers are looking for partner(s) to license and further (co-)develop and market this assay for clinical application. Specifically companies that are developing diagnostic tools for inflammatory diseases.
IP Status:
Priority patent filed
The development of therapeutics for CADASIL patients CNS, CADASIL, gene therapy, rare disease, orphan, exon skipping, oligonucleotides
CNS, CADASIL, gene therapy, rare disease, orphan, exon skipping, oligonucleotides
The development of therapeutics for CADASIL patients

Scientists at Leiden University Medical Center (LUMC) developed a potential method for the therapeutic intervention in patients suffering from CADASIL.
CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is a condition causing ischemic brain lesions, which gradually leads to cognitive decline and eventually to dementia. Currently, there is no treatment.
The disease is caused by characteristic mutations in the NOTCH3 gene resulting in an unequal number of cysteine residues and misfolding of the NOTCH3 protein.
NOTCH3 is exclusively expressed in vascular smooth muscle cells (VSMC) and this misfolding leads to an accumulation of the extracellular domain of the NOTCH3 protein and granular osmiophilic material on the surface of degenerating VSMC. In turn, this leads to impaired vascular reactivity and decreased cerebral blood flow.
Scientists at LUMC have succeeded in re-establishing an equal number of cysteine residues in the NOTCH3 protein by the exclusion of specific exons from the mRNA. They demonstrated that this reduces or even delays the accumulation of NOTCH3 on the surface of VSMC. This novel finding could lead to the development of therapeutic strategies for CADASIL patients.
Partner companies are now sought for research collaborations in this field, and licensing of key technologies. Specifically we are looking for companies with a a franchise in the treatment of CNS-ischaemic diseases.
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank obesity, metabolomic disease, biobank
obesity, metabolomic disease, biobank
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank

Interested in conducting research on obesity and metabolic disease?
Leiden University Medical Center Netherlands has finished enrollment of the Epidemiology of Obesity Study (NEO). Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers in their pursuit of causes and treatments for obesity and metabolic disease. Information ranging from health and depression questionnaires to heart and brain MRIs has been collected from 6,000 participants with a BMI = 27 kg/m2 or higher and 1,000 participants with a BMI <27 kg/m2.
Endpoints include diagnosis of Type 2 diabetes, cardiovascular disease, COPD, asthma, chronic kidney disease, osteoarthritis and all-cause mortality. Analyses were conducted on blood, serum, urine and plasma. Serum, DNA, RNA have been saved for future studies.
The database/biobank is now open for access. LUMC investigators involved in the NEO Study are also interested in research collaborations using the database/biobank.
For more information please find the link to the abstract and full publication here:
Abstract: The Netherlands Epidemiology of Obesity (NEO) study
Publication: The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection
New method for the production of the compounds with anti-cancer activity, Arglabin and Parthenolide
Wageningen University is seeking commercial partners interested in producing the anti-cancer compounds Parthenolide, Hydroxy-Parthenolide and Arglabin through microbial production platforms and/or plants expressing their biosynthesis genes.

New method for the production of the compounds with anti-cancer activity, Arglabin and Parthenolide
Wageningen University is seeking commercial partners interested in producing the anti-cancer compounds Parthenolide, Hydroxy-Parthenolide and Arglabin through microbial production platforms and/or plants expressing their biosynthesis genes.
Summary
Parthenolide and Arglabin occurs naturally in plants, such as Tanacetum parthenium (Feverfew) and Artemisia glabella (smooth Wormwood) and show strong anti-cancer activity (Guzman et. al, Blood. 2005; 105(11): 4163–4169). Dimethylaminoparthenolide is currently in phase I clinical trials and Arglabin dimethylamino adduct is a registered antitumor substance in the Republic of Kazakhstan. The lack of water-solubility and bioavailability limits so far the potential of Parthenolide as a drug.
To solubilize Parthenolide and Arglabin, extra and intensive chemical steps are needed to develop dimethylaminoparthenolide or the Arglabin derivative (Arglabin dimethylamino adduct). The lack of knowledge on the last step in the biosynthesis pathway of Parthenolide and the largely unknown biosynthesis of Arglabin blocked up to now the potential to directly produce these compounds via biotechnological production platforms, based for example on micro-organisms.
The invention
Wageningen University scientists have identified from Feverfew and Wormwood plants the key genes in the biosynthesis of Parthenolide, water-soluble Parthenolide (Hydroxy-Parthenolide) and Arglabin. When the genes are expressed in yeast and in Nicotiana benthamiana plants, both systems are able to produce de novo Parthenolide, the water-soluble Hydroxy-Parthenolide and Arglabin.Applications
Development of genetically modified plants and/or microbial production platforms for contained, continuous and direct production of the potentially anti-cancer compounds Parthenolide - in its water-soluble form - and Arglabin.
Benefits
- Provides biosynthesis genes for the production of Parthenolide in its water-soluble form and Arglabin
- May simplify the production of these potential anti-cancer compounds
- May form the basis of a continuous, efficient production process
- Production can be optimised and improved to cut costs
Stage of development
Development phase – laboratory tested. Yeast and plants were transformed with the genes and they successfully produced the target compounds.Concord: Lifesaving Care for Preterm Babies Improved by Keeping the Umbilical Cord Intact An innovative resuscitation table enabling all care needed to stabilize a preterm baby while the umbilical cord remains intact.
An innovative resuscitation table enabling all care needed to stabilize a preterm baby while the umbilical cord remains intact.
Concord: Lifesaving Care for Preterm Babies Improved by Keeping the Umbilical Cord Intact
Background
Every year, 15 million infants are born preterm worldwide. Preterm birth is responsible for over 1 million deaths each year due to complications at birth, many survivors suffer from long-term disability, including learning problems, cerebral palsy or chronic lung problems.
Most preterm infants breathe insufficiently at birth, the cord is clamped immediately to not delay the respiratory support they need to survive. However, immediate cord clamping compromises the infants' cardiovascular function, which can injure its immature organs. Waiting with cord clamping until the infant has been stabilized potentially reduces complications at birth, long term disabilities and mortality.
Technology Overview
With Concord, delayed cord clamping for preterm babies requiring lifesaving care will now be a safe option. Concord is an innovative resuscitation table that makes it possible for the neonatologist to provide all care needed to stabilize the baby, while the umbilical cord remains intact. Concord has an adjustable support bed that can be placed closely above the mother, on which the baby can be placed safely immediately after birth, to keep the sometimes very short umbilical cord intact. In addition, Concord keeps the baby close to the mother to allow bonding.
Details and State of Development:
Specialists at Leiden University Medical Center (LUMC) invented Concord and developed a clinical prototype. The prototype of Concord is used for validation of feasibility and safety in a Phase 1 clinical study. Today, 28 babies have been successfully delivered using Concord at LUMC, in the delivery room as well as in the operating room. The results are very promising regarding the feasibility and safety of the workflow, the quality of care for the baby and the very positive feedback from parents.
Concord Neonatal aims to launch a commercial product by the end of 2018. To achieve this goal, Concord Neonatal is looking for €750,000 in external investment in 3 tranches of €250,000 in 2018, 2019 and 2021.

Benefits
Many cord clamping studies compared immediate cord clamping with delayed cord clamping, focusing on breathing infants:
• Fewer babies needed blood transfusions for anemia - Relative Risk (RR) 0.61
• Reduced risk of bleeding in the brain (IVH) - RR 0.6
• Reduced risk of necrotizing enterocolitis (NEC) - RR 0.62
There is now data available showing another large benefit in delaying cord clamping until ventilation has been established. Results show that waiting with cord clamping until the lung has aerated and the infant has leg stabilized leads to placental transfusion and a more stable oxygenation of the blood and a more stable heart rate during transition. This potentially decreases the injury to the infants immature organs, especially the brains and intestines.
Further Details
Concord Neonatal BV was founded in April 2017 as a spin-out from LUMC, for the development and global commercialization of Concord.
Liposome Drug Delivery Vector Targeting the Blood Brain Barrier A lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle
A lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle
Liposome Drug Delivery Vector Targeting the Blood Brain Barrier
Background
In terms of drug delivery, the BBB is a formidable barrier. Current (pre-clinical) state-of-the-art drug delivery systems, designed to specifically target the BBB, maximally deliver <0.5% of the total injected drug dose to the brain. As such, prognoses for diseases of the brain (eg. Alzheimer’s disease, glioblastoma) remain notoriously poor.
Technology Overview
Researchers at Leiden University have developed a novel lipid, which when mixed with a naturally occurring phospholipid and formulated into 150 nm liposomes, results in a drug delivery vehicle with >10-fold selectivity for the brain endothelium (ie. the BBB) over the systemic endothelium. Encapsulation of both small molecule drugs (doxorubicin) and inorganic nanoparticles (gold nanoparticles) has been successfully demonstrated, resulting in the selective delivery of these reagents to the BBB (following intravenous injection). The novel lipid, required for BBB targeting, is synthesised in a single synthetic step (plus purification) using readily available reagents (<10 Euro/g, Sigma).
Details and State of Development:
The researchers have also demonstrated proof-of-principle of successful encapsulation of small molecule drugs as well as larger cargoes in the newly developed nanocarrier.
Applications
1. Drugs specifically targeting the brain and/or the BBB, such as treatments for strokes, cancer and neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, Huntington’s).
2. Enhancement of brain and/or BBB (theranostic) imaging.
Opportunity
All results have so far been confirmed in the embryonic zebrafish. Validation of this is technology in rodent models is currently ongoing. The researchers are looking for partners from industry/academia/SME to take this invention to the next stage: in vivo testing in mammalian models for brain-related diseas
Keywords: pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
The Development of Therapeutics for CADASIL Patients A potential method for the therapeutic intervention in patients suffering from CADASIL.
A potential method for the therapeutic intervention in patients suffering from CADASIL.
The Development of Therapeutics for CADASIL Patients
Technology Overview
Scientists at Leiden University Medical Center (LUMC) developed a potential method for the therapeutic intervention in patients suffering from CADASIL. CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is a condition causing ischemic brain lesions, which gradually leads to cognitive decline and eventually to dementia. Currently, there is no treatment. The disease is caused by characteristic mutations in the NOTCH3 gene resulting in an unequal number of cysteine residues and misfolding of the NOTCH3 protein. NOTCH3 is exclusively expressed in vascular smooth muscle cells (VSMC) and this misfolding leads to an accumulation of the extracellular domain of the NOTCH3 protein and granular osmiophilic material on the surface of degenerating VSMC. In turn, this leads to impaired vascular reactivity and decreased cerebral blood flow. Scientists at LUMC have succeeded in re-establishing an equal number of cysteine residues in the NOTCH3 protein by the exclusion of specific exons from the mRNA. They demonstrated that this reduces or even delays the accumulation of NOTCH3 on the surface of VSMC. This novel finding could lead to the development of therapeutic strategies for CADASIL patients. Data available on request: Publications,
Non-confidential presentations,
Confidential presentations.
Applications
- CADASIL treatment
- CADASIL research
Opportunity
Partner companies are now sought for research collaborations in this field, and licensing of key technologies. Specifically, the university are looking for companies with a franchise in the treatment of CNS-ischaemic diseases.
Keywords: CNS, CADASIL, gene therapy, rare disease, orphan, exon skipping, oligonucleotides
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers.
Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers.
The Netherlands Epidemiology of Obesity (NEO) Study Database and Biobank
Technology Overview
Interested in conducting research on obesity and metabolic disease?
Leiden University Medical Center Netherlands has finished enrollment of the Epidemiology of Obesity Study (NEO). Information from 6,000 obese participants from the Netherlands was gathered over a four-year period with a goal of aiding researchers in their pursuit of causes and treatments for obesity and metabolic disease. Information ranging from health and depression questionnaires to heart and brain MRIs has been collected from 6,000 participants with a BMI = 27 kg/m2 or higher and 1,000 participants with a BMI < 27 kg/m2. Endpoints include diagnosis of Type 2 diabetes, cardiovascular disease, COPD, asthma, chronic kidney disease, osteoarthritis and all-cause mortality. Analyses were conducted on blood, serum, urine and plasma. Serum, DNA, RNA have been saved for future studies.
Details and State of Development:
Four-year enrollment period is complete; follow-up is on-going.
Applications
Collaborative studies on the effect of obesity on disease Access to data and samples for in-depth studies.
Opportunity
The database/biobank is now open for access. LUMC investigators involved in the NEO Study are also interested in research collaborations using the database/biobank.
Keywords: obesity, metabolomic disease, biobank
Retractable Needle A needle and sheet system that automatically retracts when contact is made with blood.
A needle and sheet system that automatically retracts when contact is made with blood.
Retractable Needle

Background
Patients with end-stage renal disease are largely dependent on dialysis as renal replacement therapy. In haemodialysis, blood is passed through a membrane called a dialyzer, or artificial kidney ~3 times a week in order to filter out excess waste and water. The filtered blood is then returned to the body through the second needle. A hematoma (localized bleeding outside of blood vessels) resulting from incorrect needle cannulation if a very frequent problem. Major fistula infiltration occurs with ~5.2% of hemodialysis patient each year. Additionally, incorrect cannulation requires re-cannulation, which is both bad for the vessel, and results in discomfort for the patient. Moreover, 0.4% of all dialysis deaths are due to hematoma (Jose et al, 2017).
Technology Overview
A needle within a cannulation sheet has been designed that automatically retracts to a safe position when contact is made with the bloodstream. When the needle enters the vessel and blood enters the needle, a mechanism is activated which causes the needle to retract ‑ whilst leaving the sheet in place. Therefore, there is no opportunity to puncture the opposing vessel wall.
Applications
- Hemodialysis access cannulation.
- Arterial line placement.
- Pediatric venous cannulation.
Targeting ligand-bound (solid-phase) C1q We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q.
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q.
Targeting ligand-bound (solid-phase) C1q

SUMMARY
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q. This property provides unique opportunities as now these antibodies can be used to target C1q in immune complexes or C1q in specific tissues without interference of circulating C1q. Binding to circulating C1q would not only cause a huge sink for any
therapeutics or tracers, but it would also interfere with the normal C1q functions, cause clearance of C1q and reduced complement activity. Using t
BACKGROUND
C1q is the recognition molecule of the classical pathway of complement activation. C1q circulates in the blood at a concentration of around 150 μg/ml. C1q only acquires the capacity to activate the complement system after binding to an array of its ligands. These ligands include for example, immune complexes comprising antigen bound IgG antibodies or antigen bound IgM as well as ligand bound C-reactive protein. If these ligands are present in a sufficiently multimeric format than C1q binds, which results in activation of the C1 enzymes, C1r and C1s and subsequent complement activation takes place. These processes are beneficial in fighting infections and in fighting tumors, but unfortunately do also occur on host tissues where e.g. autoantibodies accumulate. Blocking C1q and classical pathway activity by antibodies has been performed, it depletes all circulating C1q and blocks classical pathway activity, however this works systemically and may put the patient in danger of infections and development of autoimmunity. Here we describe the use of very different anti-C1q autoantibodies, now targeting only ligand-bound C1q while not binding to / interfering with circulating C1q. Such anti-C1q autoantibodies have been described, also by our team, to occur in lupus patients and in some controls. They were shown to be pathogenic, but only if ligand-bound C1q was present in target organs. Obviously, the developed antibodies will be engineered to acquire the desired immune effector properties. Here we describe new, completely human antibodies that we produce recombinantly. This allows us to modify the Fc-domain of the antibody in such a way that it can no longer bind Fc-Receptors or trigger additional complement activity. In this format the antibodies can be safely used as tracers or as antibody drug conjugates. In conditions where one would like to amplify inflammation we can introduce mutations that will enhance Fc-Receptor interactions, that will boost complement activity or that may engage specific immune cells to fight cancer or (antibiotic resistant) infections.
TECHNOLOGY
The invention builds on the use of antibodies that bind to solid-phase C1q. Combined with available technologies we can generate antibodies with inert or active Fc-domains regarding immune activation. In addition we can generate tracers, and antibody-drug conjugates.
VALUE PROPOSITION
The global complement-targeted therapeutics market is expected to
grow at a CAGR of 10.5% from 2022 to 2030. The growth in this
market can be attributed to the increasing prevalence of complement-mediated diseases, rising demand for novel therapies, and growing investments in R&D by pharmaceutical companies.
TEAM
Dr. Leendert Trouw currently holds a position as Professor at the
Department of Immunology at the Leiden University Medical Center
in Leiden, The Netherlands.
Technology Offer ThermoCas9: Stable and efficient gene editing, even at high temperatures
CRISPR-Cas is a microbial adaptive immune system that uses RNA-guided nucleases to cleave foreign genetic elements. CRISPR-based genome editing tools have revolutionised fundamental research and biotechnological applications in both eukaryotes and prokaryotes due to their speed, cost-effectiveness, and precision.

Technology Offer
ThermoCas9: Stable and efficient gene editing, even at high temperatures
CRISPR-Cas is a microbial adaptive immune system that uses RNA-guided nucleases to cleave foreign genetic elements. CRISPR-based genome editing tools have revolutionised fundamental research and biotechnological applications in both eukaryotes and prokaryotes due to their speed, cost-effectiveness, and precision.
New CRISPR applications for humans, plants, animals, fungi and microbes
ThermoCas9 is a novel CRISPR-Cas9 enzyme derived from a heat-loving bacterium. Unlike the commonly used SpCas9, ThermoCas9 functions across a broad temperature range (20–65°C), making it a versatile and robust genome editing tool for use in harsh or variable conditions.
What makes ThermoCas9 unique?
- Maintains stability and activity at elevated temperatures.
- Offers high precision and stricter target matching at lower temperatures.
- New variants show improved performance at 20–37 °C — ideal for plant and human applications.
- Strong cytosine base editing activity in human cells with exceptionally wide editing window
Potential applications
- Targeted genome editing for treating human blood or metabolic disorders.
- Breeding of stress-tolerant or high-yielding crop varieties.
- Genetic engineering of microbes and fungi
- Fundamental gene function studies in plants, animals, and humans.
- Tools for nucleic acid detection and disease marker identification.
Development stage & collaboration opportunities
The test is currently between TRL 3 and 4 (proof of concept) and ready for further development. Wageningen University & Research offers access to cutting-edge tools and expertise to support further development and application.

