


A pre-targeting approach for liver radioembolisation radiation therapy, theranostics, radioembolisation, pre-targeting, interventional radiology, nuclear medicine, supramolecular chemistry, pharmaceutical industry, biotech/medical companies
radiation therapy, theranostics, radioembolisation, pre-targeting, interventional radiology, nuclear medicine, supramolecular chemistry, pharmaceutical industry, biotech/medical companies
A pre-targeting approach for liver radioembolisation

Background:
Radioembolisation is a local form of radiation-therapy that is increasingly used to treat primary liver tumours and metastases untreatable via surgery or chemotherapy. Currently, radioembolisation procedures are performed in two steps: 1) a scout-step which is used to identify (lung) shunting and to optimize dose using the non-therapeutic radiotracer 99m-technecium magroaggregate, and 2) therapeutic-step using rather costly microspheres containing therapeutic radio-isotopes (90-Ytrium or 166-Holmium).
The sequential steps are performed as two discrete procedures, usually separated because of logistical reasons by a period of two weeks. While the clinical benefit of this approach has been demonstrated, its high cost and the preclusion of procedure-related toxicity to healthy tissue e.g. lung remains a challenge. Even when using a scout scan, shunting occurs in 10% and results in the displacement of a fraction of the therapeutic microspheres outside of the diseased area, leading to ineffective dose distribution and serious adverse effects such as radiation pneumonitis. Therefore a cheaper therapeutic alternative that provides higher accuracy is needed.
Technology Overview:
Firstly, LUMC researchers have used supramolecular chemistry to develop a two-step pre-targeting approach which integrates the scout- and therapeutic-steps in a single procedure. As the therapeutic component specifically targets the diagnostic component, this prevents discrepancies in accumulation. Hence, the dose prediction becomes more accurate and the shunting issue is solved. Uniquely, the chemical interactions chosen favour complex formation within the liver, meaning that the technology is less prone to toxic side-effects due to lung shunting.
Secondly, the pre-targeting concept used creates flexibility in the therapeutic radioisotopes that can be used for the procedure. This means more easily produced radioisotopes can be used to help bring down the treatment cost. Further, the flexibility in the use of radioisotopes also allows for the creation of kit-based radioembolisation formulation that can be prepared in the hospital. With that the current therapeutic window of two weeks can be shortened to one of only hours.
Thirdly, the supramolecular chemistry used in this invention could also be adapted for use in chemoembolization approaches or for the subcutaneous needle-injection based delivery of a therapeutic dose to isolated lesions. Again, in both these indications the ability to verify the accuracy of the delivery process before administering the therapeutic component is key.
Benefits:
This technology provides a more accurate and cost effective alternative to the radioembolisation procedures currently available. The technology:
Can use a wide range of (therapeutic) radioisotopes (in addition to currently used radioisotopes), as well as chemotherapeutics, creating the potential for new markets to be explored;Improves clinical logistics by allowing one day treatments. This avoids the need for the currently used complex double procedure (scout- followed by therapeutic-procedure) spread over two weeks);Allows for kit-based radioembolisation formulations to be created and with that improves the logistics of the supply chain.
Opportunity:
We are looking for partner(s) to license the technology for commercialization to and/or to fund research into refinement and clinical translation of the technology.
Please note, header image is purely illustrative.
Source: Philip Hogeboom, NL - Wikimedia Commons - CC 3.0 Unported (CC BY 3.0)
Reconstructed Human Skin Models for Research and Screening Purposes dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
Reconstructed Human Skin Models for Research and Screening Purposes
Background:
Functional 3D reconstructed human skin equivalents (HSEs) can be used for drug testing that avoids the excessive use of experimental animals. HSEs are three-dimensional systems that recapitulate most of the in vivo characteristics and in which cellular processes may be normalized compared to conventional monolayer cultures. These in vitro 3D-HSEs are the result of more than 25 years of research and development by scientists from Leiden University Medical Centre (LUMC) (The Netherlands).
Within the Department of Dermatology, LUMC provide both healthy and diseased in vitro HSEs for animal‐free compound screening and safety testing services and for co‐development activities. HSEs are used in new product development and substantiation of product claims in cosmetic, pharmaceutical, food and environmental industries, but also in fundamental research within the field of experimental dermatology. These HSEs enable genomic, proteomic and drug development research not possible with traditional, unrepresentative or even unavailable animal models, monolayer cell cultures or clinical test procedures. Uniquely, all in vitro HSEs are fully customizable to meet both the scientific and commercial demands of the customer.
Technology Overview:
Currently LUMC offer four different HSEs; the Leiden Epidermal model (LEM), the Full-Thickness model (FTM), the Fibroblasts-Derived Matrix model (FDM) and the Ex-vivo human skin (ExHs).

(Figure 1.)
- LEM consists of keratinocytes seeded on a non-cellular matrix (e.g. inert filter membrane or de-epidermized dermis. These epidermal models are suitable for e.g. skin toxicity, irritation, or penetration tests. In 2008, the researchers pre-validated the Leiden epidermal model (LEM) for skin irritation and corrosion (El Ghalbzouri et al., 2008).
- FTM consists of keratinocytes, melanocytes and a fibroblast-populated three-dimensional collagen matrix. This model closely resembles native human skin. This full-thickness skin model can be used for tests, predictive screening and research on for example wound healing that requires the complexity of human skin, i.e. where the interaction between epidermal and dermal cells is crucial.
- FDM is similar to the FTM model, but the dermal compartment consists of human fibroblast-derived extracellular matrix. This model can be used as a tool to evaluate the effect of e.g. ingredients on dermal processes in skin aging.
- By using intact fresh skin biopsies, the researchers can perform short-term studies on human skin. By placing these skin samples onto an inert filter they can culture these ex vivo skin models (ExHs) up to 1 week.
All models can be used for predictive screening or contract research that requires the complexity of human skin. Variations of these models can be generated by incorporation of other cell types (e.g. melanocytes, different types of fibroblasts, tumour cells etc.). The different models can also be grown at different oxygen concentrations.
Within the Department of Dermatology, LUMC have exploited the HSEs for more than ten years as a tool to conduct contract research for a number or large and medium size enterprises. These projects are focused on different aspects of healthy and diseased skin, such as; skin biology, dermal interactions, skin aging, wound healing, scars, treatment of bacterial infections by antibiotics and/or anti-microbial peptides. Some examples are given in Figure 2.
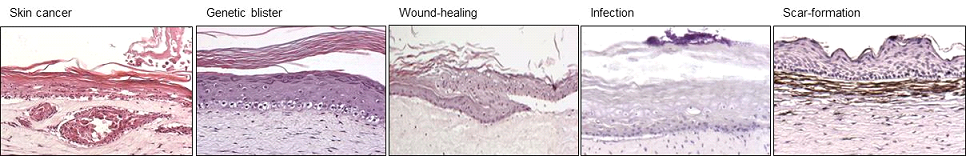
(Figure 2.)
Photo activated cancer prodrug metallodrugs, light activation, drug delivery, prodrugs, pharmaceutical industry, biotech/medical companies
metallodrugs, light activation, drug delivery, prodrugs, pharmaceutical industry, biotech/medical companies
Photo activated cancer prodrug
Technology Overview:
Researchers at Leiden University in collaboration with researchers from Texas State University have developed a novel photo activated anticancer prodrug. This prodrug consists in a rigidin analogue caged by a ruthenium polypyridyl complex that can be released upon green light irradiation. The photocaged rigidin inhibitor is a microtubule polymerization inhibitor, and as such disrupts the formation of tumor vasculature.
Microtubule-targeting agents have been used in clinic for over 50 years, but they can be very toxic for the patient. The successful caging and release upon irradiation of the inhibitor means that it is now possible to keep the systemic concentration of the (pro)drug at a higher level than the level of toxicity, without such a high concentration causing significant biological effects. By shining light at the tumour site, a high dose of the inhibitor is only released locally (or in other words, the caged inhibitor is “hidden” in the dark).
Because the agent activation is irreversible, activity will be retained after light irradiation has been removed. Also, unlike in photodynamic therapy, where oxygen concentration in the irradiated tumor should be high enough, these new compounds remain active under hypoxic conditions (1% oxygen) as their activation mechanism does not rely on the presence of dioxygen.
Finally, attachment of the ruthenium-based caging significantly increases the water solubility of the drug in the dark.
Potential applications:
- Cancer therapy
State of development:
The 7-deazahypoxanthine synthetic analogues of marine alkaloid rigidins have shown promising anticancer activities; they were found to be highly effective in eradicating cancer cells in cell cultures at low doses (double- to single-digit nanomolar antiproliferative IC50 values) and showed statistically significant tumor size reduction in a colon cancer mouse model at nontoxic concentrations.
Opportunity:
The researchers are looking for partners from industry to take this invention to the next stage, which would be to carry out extensive in vivo (mouse model) studies.
IP Status:
The rigidin analogues have been patented by New Mexico Tech and Texas State University jointly, while a patent application has been filed for the caging technology by Texas State University and Leiden University jointly.
Reconstructed Human Skin Models for Research and Screening Purposes Four human skin equivalents that can be used for predictive screening or contract research that requires the complexity of human skin.
Four human skin equivalents that can be used for predictive screening or contract research that requires the complexity of human skin.
Reconstructed Human Skin Models for Research and Screening Purposes
Background
Functional 3D reconstructed human skin equivalents (HSEs) can be used for drug testing that avoids the excessive use of experimental animals. HSEs are three-dimensional systems that recapitulate most of the in vivo characteristics and in which cellular processes may be normalized compared to conventional monolayer cultures. These in vitro 3D HSEs are the result of more than 25 years of research and development by scientists from Leiden University Medical Center (LUMC) (The Netherlands). Within the Department of Dermatology, LUMC provide both healthy and diseased in vitro HSEs for animal-free compound screening and safety testing services and for co-development activities. HSEs are used in new product development and substantiation or product claims in cosmetic, pharmaceutical, food and environmental industries, but also in fundamental research within the field of experimental dermatology. These HSEs enable genomic, proteomic and drug development research not possible with traditional, unrepresentative or equally unavailable animal models, monolayer cell cultures or clinical test procedures. Uniquely, all in vitro HSEs are fully customizable to meet both the scientific and commercial demands of the customer.
Technology Overview
Currently LUMC offer four different HSEs; the Leiden Epidermal model (LEM), the Full-Thickness model (FTM), the Fibroblasts-Derived Matrix model (FDM) and the Ex-vivo human skin (ExHs) (Figure 1).
1. LEM consists of keratinocytes seeded on a non-cellular matrix (e.g. inert filter membrane or de-epidermized dermis. These epidermal models are suitable for e.g. skin toxicity, irritation, or penetration tests. In 2008, the researchers pre-validated the Leiden epidermal model (LEM) for skin irritation and corrosion (El Ghalbzouri et al., 2008).
2 FTM consists of keratinocytes, melanocytes and a fibroblast-populated three-dimensional collagen matrix. This model closely resembles native human skin. This full-thickness skin model can be used for tests, predictive screening and research on for example wound healing that requires the complexity of human skin, i.e. where the interaction between epidermal and dermal cells is crucial.
3. FDM is similar to the FTM model, but the dermal compartment consists of human fibroblast-derived extracellular matrix. This model can be used as a tool to evaluate the effect of e.g. ingredients on dermal processes in skin aging.
4. By using intact fresh skin biopsies, the researchers can perform short-term studies on human skin. By placing these skin samples onto an inert filter they can culture these ex vivo skin models (ExHs) up to 1 week.

(Figure 1.)
All models can be used for predictive screening or contract research that requires the complexity of human skin. Variations of these models can be generated by incorporation of other cell types (e.g. melanocytes, different types of fibroblasts, tumour cells etc.). The different models can also be grown at different oxygen concentrations.
Within the Department of Dermatology, LUMC have exploited the HSEs for more than ten years as a tool to conduct contract research for a number or large and medium size enterprises. These projects are focused on different aspects of healthy and diseased skin, such as; skin biology, dermal interactions, skin aging, wound healing, scars, treatment of bacterial infections by antibiotics and/or anti-microbial peptides. Some examples are given (Figure 2).

(Figure 2.)
Applications
There is an unmet clinical need for dermatology to develop therapies for a large number of skin diseases, such as skin cancer (eg melanoma), wound healing, bacterial wound infections, and eczema. There are currently no suitable in vitro models to screen and validate novel targets, and test the effects of potential new therapies in vitro . Cosmetics, Food, Chemical, Pharmaceutical, Medical devices.
Opportunity
The models are available for contract research and for co-development activities.
Keywords: dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
A Pre-Targeting Approach for Liver Radioembolisation A two-step pre-targeting approach for radiation therapy with increased accuracy which is less prone to toxic side-effects.
A two-step pre-targeting approach for radiation therapy with increased accuracy which is less prone to toxic side-effects.
A Pre-Targeting Approach for Liver Radioembolisation
Background
Radioembolisation is a local form of radiation-therapy that is increasingly used to treat primary liver tumours and metastases untreatable via surgery or chemotherapy. Currently, radioembolisation procedures are performed in two steps: 1) a scout-step which is used to identify (lung) shunting and to optimize dose using the non-therapeutic radiotracer 99m-technecium magroaggregate, and 2) therapeutic-step using rather costly microspheres containing therapeutic radio-isotopes (90-Ytrium or 166-Holmium).The sequential steps are performed as two discrete procedures, usually separated because of logistical reasons by a period of two weeks. While the clinical benefit of this approach has been demonstrated, its high cost and the preclusion of procedure-related toxicity to healthy tissue e.g. lung remains a challenge. Even when using a scout scan, shunting occurs in 10% and results in the displacement of a fraction of the therapeutic microspheres outside of the diseased area, leading to ineffective dose distribution and serious adverse effects such as radiation pneumonitis. Therefore a cheaper therapeutic alternative that provides higher accuracy is needed.
Technology Overview
Firstly, LUMC researchers have used supramolecular chemistry to develop a two-step pre-targeting approach which integrates the scout- and therapeutic-steps in a single procedure. As the therapeutic component specifically targets the diagnostic component, this prevents discrepancies in accumulation. Hence, the dose prediction becomes more accurate and the shunting issue is solved. Uniquely, the chemical interactions chosen favour complex formation within the liver, meaning that the technology is less prone to toxic side-effects due to lung shunting. Secondly, the pre-targeting concept used creates flexibility in the therapeutic radioisotopes that can be used for the procedure. This means more easily produced radioisotopes can be used to help bring down the treatment cost. Further, the flexibility in the use of radioisotopes also allows for the creation of kit-based radioembolisation formulation that can be prepared in the hospital. With that the current therapeutic window of two weeks can be shortened to one of only hours. Thirdly, the supramolecular chemistry used in this invention could also be adapted for use in chemoembolization approaches or for the subcutaneous needle-injection based delivery of a therapeutic dose to isolated lesions. Again, in both these indications the ability to verify the accuracy of the delivery process before administering the therapeutic component is key.
Opportunity
We are looking for partner(s) to license the technology for commercialization to and/or to fund research into refinement and clinical translation of the technology.
Keywords: radiation therapy, theranostics, radioembolisation, pre-targeting, interventional radiology, nuclear medicine, supramolecular chemistry, pharmaceutical industry, biotech/medical companies
Please note, header image is purely illustrative.
Source: Philip Hogeboom, NL - Wikimedia Commons - CC 3.0 Unported (CC BY 3.0)
Photoactivatable Anticancer Prodrug A novel photo activated anticancer prodrug consisting in a rigidin analogue caged by a ruthenium polypyridyl complex
A novel photo activated anticancer prodrug consisting in a rigidin analogue caged by a ruthenium polypyridyl complex
Photoactivatable Anticancer Prodrug
Background
In oncology non-resectable, non-metastasized tumor treatment currently relies on chemotherapy, radiation therapy, or photodynamic therapy (PDT). Chemotherapy can be effective but it can be done because of side effects (neuron damage, pain, fatigue, etc.). Radiation therapy and PDT are more local and lower systemic toxicity but they require dioxygen in the irradiated tissues to be efficient. Hypoxic tumors, subset or tumors with high volume or hypoxic, poorly vascularized tissues, low prognosis for the patient.
The present invention is a form of photoactivated chemotherapy (PACT) that combines the advantages of well-defined target (PDT) and PDT (local activation by light and lower side effects). It is more specifically aimed at treating hypoxic tumors.
Technology Overview
The technology relies on the photochemical breakage of a chemical bond. In the prodrug form) a toxic, thioether-containing microtubule polymerization inhibitor is coordinated to a non-toxic ruthenium (II) caging complex. In the dark, the coordination bond between Ru 2+ and the sulfur atom is stable, but under light irradiation is broken, and the non-toxic caging group.
Details and State of Development:
- Collaboration between Leiden University, NL, and Texas State University, USA.
- Uncaged microtubule inhibitor patented by Prof. Alexander Kornienko from Texas State University, USA
- Synthesis, photochemistry, dark stability, and microtubule polymerization inhibition upon light irradiation, have been demonstrated
Chromatography-free synthesis available
- Low dark toxicity and high toxicity in vitro in 2D human cancer cell monolayers demonstrated both in normoxic (21% O 2 ) and hypoxic (1% O 2 ) conditions
- Low dark toxicity and high toxicity in vitro in A549 lung cancer 3D tumor spheroids
- Preliminary results in vivodemonstrate 30% tumor volume reduction under green light irradiation in A549 lung tumor xenografts in nude mice, and no toxicity in the dark, after intraperitoneal injection at 1 mg / kg and with a green light dose of 38 J / cm 2 .
Applications
Market for photoactivated chemotherapy includes brain, liver, head and neck, non-melanoma skin, and eye cancer (Market study available). Best application for tumors with a high ratio of hypoxic to normoxic volume, for which currently available therapies (PDT, radiation, surgery) do not work or are impossible (non-resectable tumors).
Opportunity
The researchers are looking for partners in the field of research, which would be extensive in vivo (mouse model) studies.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE:
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE:
Summary
Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
Background
HD is a rare neurodegenerative genetic disorder caused by an abnormal expansion of a CAG triplet repeat in the HTT gene. This results in a mutant huntington protein causing the disease. Current approved treatments focus on symptoms rather than the underlying genetic cause. Proteolytic cleavage of the full length protein into smaller and more toxic N-terminal fragments are an important step in HD pathology. Targeting these cleavage sites in HTT RNA directly offers a promising path for altering the disease course, considering the essential roles of the normal huntington protein.
Technology
Our ASO therapy involves splice modulation of HTT pre-mRNA, employing a strategy that targets exon 12 of both the wild-type and mutant HTT. This approach creates a caspase-6 resistant HTT protein (Htt∆12), reducing the toxicity of the mutant protein. This method importantly preserves enough HTT function, crucial for brain health and homeostasis, as complete silencing of HTT can affect motor function, anxiety behavior, and survival.
Research and Development
Extensive preclinical studies conducted in YAC128 Huntington mice have demonstrated the efficacy of the ASO therapy. Key findings include:
- Improved phenotypes: treated mice exhibited significant improvements in body weight, activity levels, and reduced loss of striatal volume
- Gene expression and protein level changes: post-treatment analyses revealed a trend toward normalization of striatal gene expression and protein levels, moving closer to wild-type levels
Value proposition
Our ASO therapy for HD offers a transformative treatment option with potential benefits including:
- Targeted Genetic Therapy: direct targeting of the HTT gene mutation, offering a more effective treatment strategy compared to current symptomatic treatments.
- Preservation of Normal HTT Function: unique exon skipping approach that maintains essential HTT functions, crucial for brain health.
- Strong Preclinical Efficacy: demonstrated functional efficacy in animal models, setting a solid foundation for human clinical trials.
Market opportunity
The Huntington’s disease is a rare disease with prevalence of 7-8 per 100,000 in Europe and North America. There are currently no treatments available that slow or reverse the disease. The HD treatment market is projected to expand significantly, from USD 380 million in 2022 to an estimated USD 2018 million by 2030, at a CAGR of 23.20% (Grand View Research).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team at the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology, ensuring a comprehensive and innovative approach to HD treatment. There are strong links to the Neurology department at the LUMC where dr. Susanne de Bots leads the largest outpatient clinic of the Netherlands with around 500 HD patient visits per year.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE:
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE:
Summary
Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
Background
D-CAA, (Dutch type cerebral amyloid angiopathy (CAA), also known as HCHWA-D or Katwijk’s disease), is caused by a point mutation in the APP gene, leading to brain bleeds and early fatality. Similarly, certain APP mutations and increased production of the harmful amyloid-beta peptide are implicated in AD. Current treatment options are limited, emphasizing the need for targeted genetic therapies.
Technology
The ASO therapy focuses on splice modulation, specifically targeting APP exon 17. This results in an APP protein lacking part of the amyloid-beta peptide sequence, thereby reducing the formation of harmful amyloid peptides.
In vitro studies and Molecular Mechanism
- Initial in vitro studies in human cell lines demonstrated the ASO therapy’s ability to induce exon 17 skipping in the APP gene, leading to an APP protein variant without the amyloid-beta peptide, a key factor in plaque formation.
- Molecular analysis confirmed the ASO's targeted action in altering APP mRNA, showcasing its precision and specificity.
Preclinical Studies in iPSC Models
- Preclinical research in patient-derived human induced pluripotent stem cells (iPSC) showed the ASO therapy's effectiveness in reducing amyloid-beta levels in vitro, a major pathological marker in D-CAA and Alzheimer's Disease, suggesting the therapy’s potential to counteract neurodegeneration.
Safety and Efficacy
- The ASO therapy maintained a favorable safety profile throughout preclinical studies, with no significant adverse effects in treated models showing no overt toxicity at the highest delivered dose.
Value proposition
This ASO therapy targets APP and is applicable to all diseases with harmful amyloid-beta peptide accumulation, such as D-CAA and Alzheimer's Disease, potentially reducing amyloid-beta formation and altering disease progression. Its application extends beyond these conditions, addressing significant unmet needs in various neurodegenerative diseases.
The Alzheimer's disease market alone is expected to grow significantly, with a projected compound annual growth rate of 20.0%, increasing from $2.2 billion in 2020 to $13.7 billion by 2030 (Global Data).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team of the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR HUNTINGTON’S DISEASE

Summary
Leiden University Medical Center's NeuroD research group has developed an antisense oligonucleotide (ASO) therapy for Huntington's Disease (HD), targeting the disease-causing HTT transcript. This novel therapy aims to reduce the toxicity of mutant HTT protein while preserving the vital functions of the normal protein, and has demonstrated improvement in symptoms in an HD mouse model, marking a significant advance in HD treatment.
Background
HD is a rare neurodegenerative genetic disorder caused by an abnormal expansion of a CAG triplet repeat in the HTT gene. This results in a mutant huntington protein causing the disease. Current approved treatments focus on symptoms rather than the underlying genetic cause. Proteolytic cleavage of the full length protein into smaller and more toxic N-terminal fragments are an important step in HD pathology. Targeting these cleavage sites in HTT RNA directly offers a promising path for altering the disease course, considering the essential roles of the normal huntington protein.
Technology
Our ASO therapy involves splice modulation of HTT pre-mRNA, employing a strategy that targets exon 12 of both the wild-type and mutant HTT. This approach creates a caspase-6 resistant HTT protein (Htt∆12), reducing the toxicity of the mutant protein. This method importantly preserves enough HTT function, crucial for brain health and homeostasis, as complete silencing of HTT can affect motor function, anxiety behavior, and survival.
Research and Development
Extensive preclinical studies conducted in YAC128 Huntington mice have demonstrated the efficacy of the ASO therapy. Key findings include:
- Improved phenotypes: treated mice exhibited significant improvements in body weight, activity levels, and reduced loss of striatal volume
- Gene expression and protein level changes: post-treatment analyses revealed a trend toward normalization of striatal gene expression and protein levels, moving closer to wild-type levels
Value proposition
Our ASO therapy for HD offers a transformative treatment option with potential benefits including:
- Targeted Genetic Therapy: direct targeting of the HTT gene mutation, offering a more effective treatment strategy compared to current symptomatic treatments.
- Preservation of Normal HTT Function: unique exon skipping approach that maintains essential HTT functions, crucial for brain health.
- Strong Preclinical Efficacy: demonstrated functional efficacy in animal models, setting a solid foundation for human clinical trials.
Market opportunity
The Huntington’s disease is a rare disease with prevalence of 7-8 per 100,000 in Europe and North America. There are currently no treatments available that slow or reverse the disease. The HD treatment market is projected to expand significantly, from USD 380 million in 2022 to an estimated USD 2018 million by 2030, at a CAGR of 23.20% (Grand View Research).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team at the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology, ensuring a comprehensive and innovative approach to HD treatment. There are strong links to the Neurology department at the LUMC where dr. Susanne de Bots leads the largest outpatient clinic of the Netherlands with around 500 HD patient visits per year.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
ANTISENSE OLIGONUCLEOTIDE THERAPY FOR D-CAA AND ALZHEIMER'S DISEASE
Summary
Leiden University Medical Center's NeuroD research group has developed an innovative antisense oligonucleotide (ASO) therapy targeting APP gene mutations, applicable to D-CAA and Alzheimer's Disease (AD). This therapy is designed to modulate APP pre-mRNA splicing, reducing harmful amyloid-beta production.
Background
D-CAA, (Dutch type cerebral amyloid angiopathy (CAA), also known as HCHWA-D or Katwijk’s disease), is caused by a point mutation in the APP gene, leading to brain bleeds and early fatality. Similarly, certain APP mutations and increased production of the harmful amyloid-beta peptide are implicated in AD. Current treatment options are limited, emphasizing the need for targeted genetic therapies.
Technology
The ASO therapy focuses on splice modulation, specifically targeting APP exon 17. This results in an APP protein lacking part of the amyloid-beta peptide sequence, thereby reducing the formation of harmful amyloid peptides.
In vitro studies and Molecular Mechanism
- Initial in vitro studies in human cell lines demonstrated the ASO therapy’s ability to induce exon 17 skipping in the APP gene, leading to an APP protein variant without the amyloid-beta peptide, a key factor in plaque formation.
- Molecular analysis confirmed the ASO's targeted action in altering APP mRNA, showcasing its precision and specificity.
Preclinical Studies in iPSC Models
- Preclinical research in patient-derived human induced pluripotent stem cells (iPSC) showed the ASO therapy's effectiveness in reducing amyloid-beta levels in vitro, a major pathological marker in D-CAA and Alzheimer's Disease, suggesting the therapy’s potential to counteract neurodegeneration.
Safety and Efficacy
- The ASO therapy maintained a favorable safety profile throughout preclinical studies, with no significant adverse effects in treated models showing no overt toxicity at the highest delivered dose.
Value proposition
This ASO therapy targets APP and is applicable to all diseases with harmful amyloid-beta peptide accumulation, such as D-CAA and Alzheimer's Disease, potentially reducing amyloid-beta formation and altering disease progression. Its application extends beyond these conditions, addressing significant unmet needs in various neurodegenerative diseases.
The Alzheimer's disease market alone is expected to grow significantly, with a projected compound annual growth rate of 20.0%, increasing from $2.2 billion in 2020 to $13.7 billion by 2030 (Global Data).
Team
The development of this therapy is led by Prof. Dr. Willeke van Roon-Mom and her team of the NeuroD research group in the LUMC. The team combines expertise in molecular neuroscience, genetic therapies, and clinical neurology.

