

Mouse models of spontaneous thrombosis animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
animal model, mouse model, thrombosis, atherothrombosis, venous thrombosis
Mouse models of spontaneous thrombosis
Background:
Thrombosis comes in two flavours:
- VT: Venous thrombosis (with pulmonary embolism as possible results)
- AT: Arterial thrombosis (with myocardial infarction or stroke as possible result)
Venous and arterial thrombosis are a major source of morbidity and mortality worldwide and both are complex vascular diseases for which pathogenesis is incompletely understood. Animal models are fundamental in our effort to understand the disease and develop better therapy (“Holy Grail” an antithrombotic without bleeding risk as side-effect).
Currently there are limited (venous thrombosis) or no (arterial thrombosis) technically reproducible or clinically relevant mouse models for these diseases.
Technology Overview:
Researchers at the LUMC have developed a mouse model for VT via “humanizing” mouse coagulation via RNAi of the hepatic antithrombin (Serpinc1) and protein C (Proc) genes.
In addition, they have developed a mouse model for AT via RNAi of Proc in Apoe-/- mice. In initial studies organized and large thrombi superimposed on an aortic root atherosclerotic plaque were observed, a unique and novel finding. This model needs further optimization.
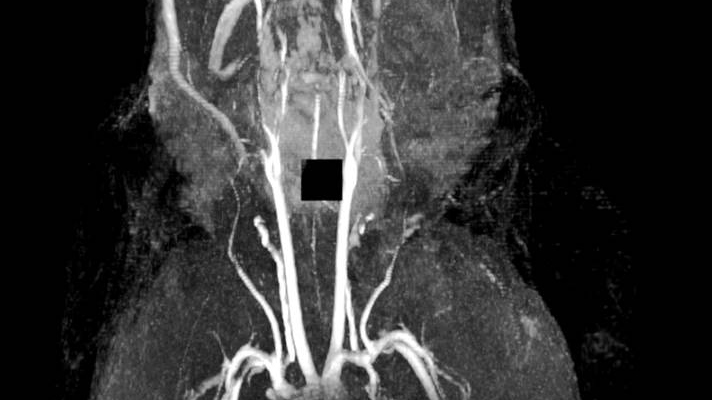
Figure 1: MRI of spontaneous venous thrombosis in a large vessel in the head (mandibular area)
Benefits:
The VTE model:
- Generates highly reproducible acute venous thrombosis
- Is technically simple and fast
- Reproduces morphologically venous thrombosis in humans
- Is responsive to (pharmacological) thrombin and platelet inhibition
- Is used by LUMC researchers and others to study VT pathogenesis
The AT model:
- Is technically simple and fast (i.e. the thrombosis part)
- Is reproducible, but has a low incidence (0.16)
- Responsiveness to drugs currently used to prevent AT unknown
- The occurrence of spontaneous atherothrombosis in the siProc apoE-/- mice is a truly unique event so far lacking in other preclinical models.
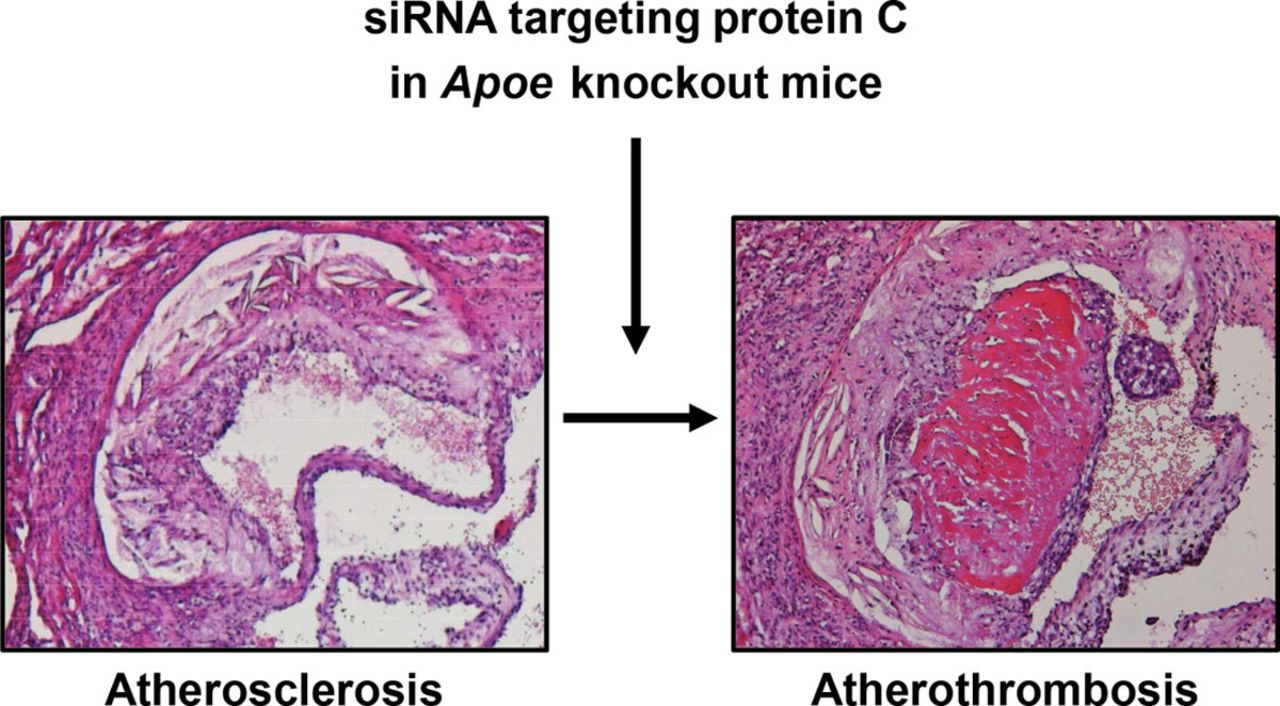
[Figure 2: Arterial (athero)thrombosis in apoliporotein E deficient mouse following RNAi]
Further Details:
VTE model: Heestermans et al., Blood. 2016 May 26;127(21):2630-7
AT model: Ouweneel et al., Arterioscler Thromb Vasc Biol. 2017 May;37(5):782-785
Applications:
Potential applications would be in preclinical research of venous thrombosis and pulmonary embolism, and arterial thrombosis and myocardial infarction and stroke.
Venous Thrombosis and Pulmonary Embolism (source: World Thrombosis Day 2016)
- Every year, there are approximately 10 million cases of VTE worldwide
- In the U.S., there are 100,000 - 300,000 VTE-related deaths every year
- In Europe, there are 544,000 VTE-related deaths every year
- Up to 60 percent of VTE cases occur during or after hospitalization, making it a leading preventable cause of hospital death.
Arterial Thrombosis and Myocardial Infarction and Stroke (source Netherlands Heart Foundation)
- Every day over 100 related deaths in The Netherlands
- Every day over 1000 cases hospitalized in The NetherlandsOver 1 million cases in The Netherlands
Opportunity:
Know-how on the VTE model is available for partnering and/or licensing.
LUMC are seeking co-development partners to further optimize the AT model and/or study its response to drugs that are currently used in MI/stroke (lipid-lowering/antiplatelet drugs) and to those in current pipelines (PAR inhibitors, FXI inhibitors, others).
Novel Pharmacologial Chaperones for Improved Enzyme Replacement Therapy A small molecule with potential to be used as part of a new treatment for Fabry patients in combination with enzyme replacement therapy.
A small molecule with potential to be used as part of a new treatment for Fabry patients in combination with enzyme replacement therapy.
Novel Pharmacologial Chaperones for Improved Enzyme Replacement Therapy
Background
Enzyme replacement therapy (ERT) is a very costly therapeutic treatment. The costs of ERT starting in the symptomatic stage are between €9 - €10 million (£ 7.9 - £ 8.8 million, $13.0- $14.5 million) during a patient's lifetime. The combination of small pharmacological chaperones (PCs) with ERT has shown a synergic effect in improved enzyme activity and reduction of toxic metabolites. Therefore, it is expected that the co-treatment PC-ERT may reduce the amount of ERT necessary to achieve the desired pharmacological effect and therefore may lower lysosomal storage diseases (LSDs) treatment costs.
Technology Overview
Leiden University researchers have developed a new class of pharmacological chaperones (PCs) named cyclosulfamidates. α-galactose configured cyclosulfamidate reacts reversibly with α-gal A and stabilized the enzyme in vitro and in situ. They have shown that α-cyclosulfamidate is able to stabilize the recombinant human enzyme in vitro and in situ cell experiments and increased α-gal A activity is observed in the medium of the cells co-treated with PC and recombinant α-gal A. This increase is translated in a slightly higher activity in the cells treated with ERT and PC combination for 24 h and 4 days.
The researchers also quantified the amount of Gb3 and lysoGb3 in treated and untreated cultured fibroblast lysates and they interestingly observed that the combination of ERT and α-cyclosulfamidate has a similar effect that ERT effect when compared to ERT alone. In conclusion, herein they describe the potential of a new small molecule, α-cyclosulfamidate, as stabilizer of α-gal A and its potential as new treatment for Fabry patients in combination with ERT.
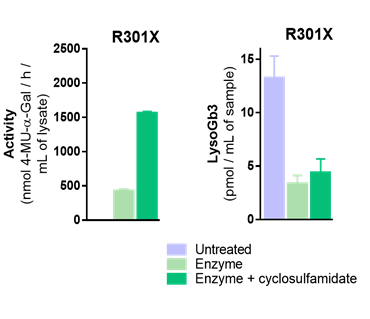 A structure activity relationship (SAR) on the galactose configured cyclosulfamidate is ongoing. In addition, glucose configured cyclosulfamidates as new potential pharmacological chaperones for Pomple and Gaucher Disease are comparatively studied.
A structure activity relationship (SAR) on the galactose configured cyclosulfamidate is ongoing. In addition, glucose configured cyclosulfamidates as new potential pharmacological chaperones for Pomple and Gaucher Disease are comparatively studied.
Applications
This invention provides various combinations of enzyme replacement therapy or (multi)gene therapies with a pharmacological chaperone for the treatment of lysosomal storage diseases and glycosidase deficiency related diseases, particularly Fabry, Gaucher or Pompe disease. This combination optimizes the clinical benefit, i.e., reduced enzyme treatment, while minimizing disadvantages associated with ERT.
Novel alpha-Glucosidase and alpha-Galactosidase Inhibitors as Antidiabetic, Antiviral Drugs and as Pharmacological Chaperones for Pompe Disease Small molecules that react reversibly or irreversibly with human α-glucosidases depending on nitrogen position of the cyclic sulfamidate.
Small molecules that react reversibly or irreversibly with human α-glucosidases depending on nitrogen position of the cyclic sulfamidate.
Novel alpha-Glucosidase and alpha-Galactosidase Inhibitors as Antidiabetic, Antiviral Drugs and as Pharmacological Chaperones for Pompe Disease
Background
The global α-glucosidase inhibitors market generated $ 4069 million in 2018 and is expected to grow at a CAGR of 2% during 2019-2024. Acarbose, Miglitol, and Voglibose are the most prescribed alpha-glucosidase inhibitors used in the management of hyperglycemia for the treatment of type 2 diabetes mellitus. On the other hand, the combination of small α-glucosidase inhibitors as pharmacological chaperones (PCs) with enzyme replacement therapy (ERT) has shown a synergic effect in improved enzyme activity and reduction of toxic metabolites. Therefore, it is expected that the co-treatment PC-ERT may reduce the amount of ERT necessary to achieve the desired pharmacological effect and therefore may lower lysosomal storage diseases (LSDs) treatment cost. Current ERT is very costly, ranging between € 9 - € 10 million (£ 7.9 - £ 8.8 million, $ 13.0- $ 14. 5 million) during a patient's lifetime. No PC-ERT treatment for Pompe disease exists. Last but not least, recent studies have demonstrated that
ER α-glucosidases I and II are essential for the morphorgenesis of many enveloped viruses, and various iminosugar-based ER α-glucosidase inhibitors has shown in vivo antiviral efficacy in animals infected with Dengue, Ebola and influenza viruses.
Technology Overview
Leiden University have developed a new class of α-glucosidase inhibitors names α-cyclosulfamidates.
α-Glucosidase configured cyclosumaidates react reversibly or irreversibly with diverse human α-glucosidases depedning on the nitrogen position of the cyclic sulfamidate. They have shown that the reversible α-cyclosulfamidate is able to stabilize the recombinant lysosomal human α-glucosidase (GAA) in in vitro and in situ cell experiments and increased enzyme activity is observed in the medium of the cells co-treated with this α- cyclosulfamidate and recombinant GAA. Herein they describe the potential treatment of Diabetes and / or viral infections, as well as the potential of the reversible analogue as stabilizer of GAA for the treatment for Pompe patients in combination with ERT.
Applications
This invention provides a set of new α-glucosidase and α-galactosidase inhibitors. α-Glucosidase inhibitors have shown beneficial effects in multiple applications (antidiabetics, antiviral and as pharmacological chaperones for Pompe disease). Various combinations of enzyme replacement therapy or (mult) gene therapies with a pharmacological chaperone (glycosidase inhibitor) for the treatment of lysosomal storage diseases and glycosidase deficiency related diseases, particularly Fabry, Gaucher or Pompe disease. This combination optimizes the clinical benefit, ie, reduced enzyme treatment, while minimizing disadvantages associated with ERT.

