




Liposome drug delivery vector targeting the blood brain barrier pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
Liposome drug delivery vector targeting the blood brain barrier
Technology Overview:
Researchers at Leiden University have developed a novel lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle with a selectivity for the brain endothelium (the blood brain barrier or BBB) of >10-fold over the systemic endothelium. This means that not <1% percent of the injected dose gets delivered to the brain and/or BBB, as is now the case with doxorubicin-filled liposomes, but potentially a 10-fold selectivity for brain endothelium over systemic endothelium or more of a drug can be delivered directly to the brain and/or BBB.
Potential Applications:
Drugs specifically targeting the brain and/or the BBB, such as treatments for strokes, cancer and neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, Huntington’s).Enhancement of brain and/or BBB (theranostic) imaging.
State of Development:
The BBB-selectivity was shown in zebrafish, that have a genome which is 70% homologous to humans and show a very similar brain morphology, organization and expression of key markers for BBB-function and integrity. Experiments in mammalian models are currently being undertaken.
The researchers have also demonstrated proof-of-principle of successful encapsulation of small molecule drugs as well as larger cargoes in the newly developed nanocarrier.
A direct, high throughput assay for Neutrophil extracellular traps (NETs) pharmaceutical industry, biotech/biomedical companies, autoimmune diseases
pharmaceutical industry, biotech/biomedical companies, autoimmune diseases
A direct, high throughput assay for Neutrophil extracellular traps (NETs)

Background:
Neutrophil extracellular traps (NETs) are immunogenic, extracellular DNA structures that harness important auto-antigens to be recognized by the adaptive immune system. Recent evidence suggests that NETs have a role in a number of noninfectious diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), ANCA-associated vasculitis (AAV), diabetes, atherosclerosis and cancer. However, it is still unclear how and if NETs act as a common pathway in the pathophysiology of these clinically divergent autoimmune diseases. The exact role of NETs in these diseases remains to be elucidated and one limiting factor has been the lack of a well-defined assay to quantify NET formation. NETs are thought to play a role in the initiation of many noninfectious conditions, and, in combination with imaging NET production, this opens up the possibility of new therapies.
Technology Overview:
Researchers at LUMC have developed a direct, high throughput assay to quantifying NETs and are using this assay to study SLE and AAV patients. The assay directly visualizes and quantifies the amount of NETs produced on any given stimulus. The group at LUMC has a particular interest in autoimmune diseases, such as AAV and SLE). Any autoimmune diseases consist of periods of remission followed by episodes of disease activity (more about research and groups expertise can be found at http://www.einthovenlaboratory.com).
Benefits:
As the assay combines immunohistochemistry with quantification of extracellular DNA it provides an accurate assay that can be scaled up for high throughput.
Potential applications
This assay could be used as
- Diagnostic/ predictive test
- Clinical test of disease activity
- Ex vivo test for screening potential drugs
Further background:
http://www.nature.com
High throughput 3D cell culture assay 3D cell culture, cell spheroids, high-throughput, drug screening, breast cancer, cancer
3D cell culture, cell spheroids, high-throughput, drug screening, breast cancer, cancer
High throughput 3D cell culture assay

Scientist at Leiden University have developed a fast and robust 3-dimensional (3D) cell culture assay.
In this 3D assay, cell speroids are formed within minutes at precise pre-determined positions.This novel 3D cell culture assay is highly suited for high throughput drug screening, especially in cancer research.
The need for better in vitro screening technology is well known and documented in the field. The currently used 2D cell assays are fast and easy to perform, but lack predictive value. Among the various 3D culture platforms, 3D ECM-embedded cell spheroids most closely represent the in vivo tumor microenvironments. However, several technical hurdles preclude the use of such cultures in high content screening (HCS): i) spheroid creation is difficult and poorly reproducible: ii) it is restricted to certain cell types: and iii) spheroid location cannot be carefully controlled, which hampers automated imaging.
Scientists at Leiden University have developed an automated method to create 3D ECM-embedded cell spheroids that overcomes these limitations. Spheroid formation time is strongly reduced compared to other methods (minutes rather than days) and it can be applied to a broad range of cell types including cells which naturally do not form cell-cell contacts, endothelial cells, various cancer cell lines, and primary tumor cell suspensions. For High Content Screening, we are able to produce 1 spheroid per well in 384 well plates or up to 7 patterned spheroids per well in 96 well plates. Importantly, the spheroids have defined x-y-z spatial coordinates allowing automated confocal imaging and image analysis algorithms. A successful proof-of-principle chemical screen was done on breast cancer spheroids identifying compounds affecting cancer cell invasion/migration.

New mouse model, expressing human Fc receptors, to test the efficacy of therapeutic antibodies drug delivery, dermatology, cosmetics, antibodies, collaboration
drug delivery, dermatology, cosmetics, antibodies, collaboration
New mouse model, expressing human Fc receptors, to test the efficacy of therapeutic antibodies
Over the years, a lot of therapeutic antibodies have been developed and marketed. These antibodies have the capacity to treat diseases such as rheumatoid arthritis, multiple sclerosis, psoriasis and several forms of cancer.
The potential of antibodies for healthcare is enormous and several new antibodies directed to new targets are under development. It appears very difficult to test the efficacy of the therapeutic antibodies in the first phases of clinical research. This is currently performed in vitro and in mouse models. However, in vitro tests do not represent the broad context of the body, whereas in the mouse the Fc receptors (FcR), which are the main molecules interacting with antibodies, are not identical to human FcR. For this reason there is a strong need for more reliable in vivo test models based on the characteristics of the human body.
To fulfill this need, scientists at Leiden University Medical Center have developed two new mouse models, which, when crossed, will generate a mouse model with human FcR in the absence of mouse FcR. (‘Humanized mouse’). The scientists are looking for a commercial partner to develop the desired mouse model to be able to study the effects of therapeutic antibodies in the human body.
Furthermore, since the copy number and expression of the different members of the FcR family varies between individuals, additional models could be developed, displaying specific types of human FcR, which will then be representative for a specific part of the population. The availability of this kind of models will allow for the development of personalized therapeutic strategies, dependent on the patient’s FcR expression. We are looking for a biotechnology/pharmaceutical partner with experience in drug testing and/or the development of therapeutic antibodies.
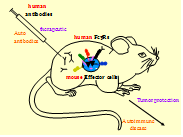
Fig. 1 The in vivo test tube for the validation of human therapeutic monoclonal antibodies and the analysis of human pathogenic antibodies
Adjuvant Compounds for Conjugated Synthetic Vaccines adjuvant, agonist, cell, activation, immune response, immunological ligands, synthetic vaccine
adjuvant, agonist, cell, activation, immune response, immunological ligands, synthetic vaccine
Adjuvant Compounds for Conjugated Synthetic Vaccines
Background
Synthetic vaccines consist of well-defined chemically produced molecules and require two components: an antigen and an immune-stimulating adjuvant. In peptide-based synthetic vaccines the antigen is a peptide that contains a sequence (the epitope) that is specifically recognized by the T cell immune system while the adjuvant is often a ligand of one of Toll-like receptors (TLR’s) that provides a “danger signal” and expands specific T cells. In this invention an improved adjuvant is described that can be used as the entity covalently attached to any synthetic peptide sequence.
Tech Overview
This technology builds on a well-known design of conjugated vaccines comprising synthetic lipopeptides, in which the lipophilic TLR-2 agonist is attached to the N-terminus of synthetic antigenic peptide. Researchers from Leiden University have previously shown that classical TLR2 ligand-conjugated peptide vaccines has strong T cell activation potency in vivo and can control aggressive tumor growth. The compound contains a defined lipidcomponent which can be covalently attached to any synthetic peptide sequence. The adjuvant compound retains its strong adjuvanticity upon conjugation but possesses much improved physicochemical properties as compared to established lipid-based synthetic vaccines. The synthesis of the conjugatable TLR-2 agonist is accomplished by the methods of solution phase organic chemistry and subsequently it is readily coupled to a peptide sequence preassembled on solid-phase by standard method of peptide chemistry. The finished conjugate is liberated from the solid-phase and purified by the techniques common in the preparation of peptide-based vaccines.
The conjugable TLR2 lipopeptide adjuvant platform is suited to augment the immunogenicity of synthetic peptidebased vaccines to any pathogen or malignancy of which sequence information of immunogenic epitopes is
available. Especially for personalized vaccines for cancer patients this adjuvant offers a flexible system to swiftly conjugate multiple peptide sequences of tumor-specific mutated neo-epitopes.
New method for the production of the compounds with anti-cancer activity, Arglabin and Parthenolide
Wageningen University is seeking commercial partners interested in producing the anti-cancer compounds Parthenolide, Hydroxy-Parthenolide and Arglabin through microbial production platforms and/or plants expressing their biosynthesis genes.

New method for the production of the compounds with anti-cancer activity, Arglabin and Parthenolide
Wageningen University is seeking commercial partners interested in producing the anti-cancer compounds Parthenolide, Hydroxy-Parthenolide and Arglabin through microbial production platforms and/or plants expressing their biosynthesis genes.
Summary
Parthenolide and Arglabin occurs naturally in plants, such as Tanacetum parthenium (Feverfew) and Artemisia glabella (smooth Wormwood) and show strong anti-cancer activity (Guzman et. al, Blood. 2005; 105(11): 4163–4169). Dimethylaminoparthenolide is currently in phase I clinical trials and Arglabin dimethylamino adduct is a registered antitumor substance in the Republic of Kazakhstan. The lack of water-solubility and bioavailability limits so far the potential of Parthenolide as a drug.
To solubilize Parthenolide and Arglabin, extra and intensive chemical steps are needed to develop dimethylaminoparthenolide or the Arglabin derivative (Arglabin dimethylamino adduct). The lack of knowledge on the last step in the biosynthesis pathway of Parthenolide and the largely unknown biosynthesis of Arglabin blocked up to now the potential to directly produce these compounds via biotechnological production platforms, based for example on micro-organisms.
The invention
Wageningen University scientists have identified from Feverfew and Wormwood plants the key genes in the biosynthesis of Parthenolide, water-soluble Parthenolide (Hydroxy-Parthenolide) and Arglabin. When the genes are expressed in yeast and in Nicotiana benthamiana plants, both systems are able to produce de novo Parthenolide, the water-soluble Hydroxy-Parthenolide and Arglabin.Applications
Development of genetically modified plants and/or microbial production platforms for contained, continuous and direct production of the potentially anti-cancer compounds Parthenolide - in its water-soluble form - and Arglabin.
Benefits
- Provides biosynthesis genes for the production of Parthenolide in its water-soluble form and Arglabin
- May simplify the production of these potential anti-cancer compounds
- May form the basis of a continuous, efficient production process
- Production can be optimised and improved to cut costs
Stage of development
Development phase – laboratory tested. Yeast and plants were transformed with the genes and they successfully produced the target compounds.Reconstructed Human Skin Models for Research and Screening Purposes dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
Reconstructed Human Skin Models for Research and Screening Purposes
Background:
Functional 3D reconstructed human skin equivalents (HSEs) can be used for drug testing that avoids the excessive use of experimental animals. HSEs are three-dimensional systems that recapitulate most of the in vivo characteristics and in which cellular processes may be normalized compared to conventional monolayer cultures. These in vitro 3D-HSEs are the result of more than 25 years of research and development by scientists from Leiden University Medical Centre (LUMC) (The Netherlands).
Within the Department of Dermatology, LUMC provide both healthy and diseased in vitro HSEs for animal‐free compound screening and safety testing services and for co‐development activities. HSEs are used in new product development and substantiation of product claims in cosmetic, pharmaceutical, food and environmental industries, but also in fundamental research within the field of experimental dermatology. These HSEs enable genomic, proteomic and drug development research not possible with traditional, unrepresentative or even unavailable animal models, monolayer cell cultures or clinical test procedures. Uniquely, all in vitro HSEs are fully customizable to meet both the scientific and commercial demands of the customer.
Technology Overview:
Currently LUMC offer four different HSEs; the Leiden Epidermal model (LEM), the Full-Thickness model (FTM), the Fibroblasts-Derived Matrix model (FDM) and the Ex-vivo human skin (ExHs).

(Figure 1.)
- LEM consists of keratinocytes seeded on a non-cellular matrix (e.g. inert filter membrane or de-epidermized dermis. These epidermal models are suitable for e.g. skin toxicity, irritation, or penetration tests. In 2008, the researchers pre-validated the Leiden epidermal model (LEM) for skin irritation and corrosion (El Ghalbzouri et al., 2008).
- FTM consists of keratinocytes, melanocytes and a fibroblast-populated three-dimensional collagen matrix. This model closely resembles native human skin. This full-thickness skin model can be used for tests, predictive screening and research on for example wound healing that requires the complexity of human skin, i.e. where the interaction between epidermal and dermal cells is crucial.
- FDM is similar to the FTM model, but the dermal compartment consists of human fibroblast-derived extracellular matrix. This model can be used as a tool to evaluate the effect of e.g. ingredients on dermal processes in skin aging.
- By using intact fresh skin biopsies, the researchers can perform short-term studies on human skin. By placing these skin samples onto an inert filter they can culture these ex vivo skin models (ExHs) up to 1 week.
All models can be used for predictive screening or contract research that requires the complexity of human skin. Variations of these models can be generated by incorporation of other cell types (e.g. melanocytes, different types of fibroblasts, tumour cells etc.). The different models can also be grown at different oxygen concentrations.
Within the Department of Dermatology, LUMC have exploited the HSEs for more than ten years as a tool to conduct contract research for a number or large and medium size enterprises. These projects are focused on different aspects of healthy and diseased skin, such as; skin biology, dermal interactions, skin aging, wound healing, scars, treatment of bacterial infections by antibiotics and/or anti-microbial peptides. Some examples are given in Figure 2.
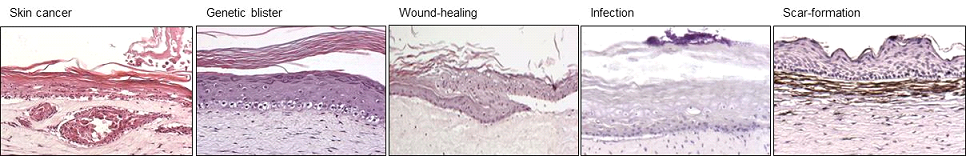
(Figure 2.)
Platinum metal-based anticancer and antibacterial drugs metallodrugs, bacteria, cancer, pharmaceutical industry, biotech/medical companies
metallodrugs, bacteria, cancer, pharmaceutical industry, biotech/medical companies
Platinum metal-based anticancer and antibacterial drugs
Technology Overview:
Researchers at Leiden University have developed novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents. A family of related ligands can be used in the formulation of the compounds, to tune solubility and activity. The compounds are very stable due to the tetradentate nature of the ligands, coupled with a tetracoordinated (d8) metal center.
While these novel compounds are both simple and cheap to synthesize, they could provide an interesting new alternative for platinum-based anticancer drugs (platinum based drugs such as cisplatin, oxaliplatin and carboplatin already have FDA-approval and are used widely). These new compounds are all the more interesting, because micromolar to nanomolar activities have been observed on cancer cell lines that are resistant to cisplatin.
However the activities are not limited to cancer therapy; applications as an antibacterial agent are also possible. The exact activities of the different compounds depend on the entire compound, while the mode of action depends critically on the nature of the metal.
Potential applications:
- Cancer therapy
- Antibacterial agent
State of development:
The compounds have been synthesized and characterized. In vitro and in vivo (mouse model) experiments have been conducted that have confirmed the high potential of the compounds; in vitro experiments have revealed that the cytotoxicity of some derivatives on human cancer cell lines are among the highest ever reported in the literature, while preliminary in vivo experiments in a mouse model show a decrease in tumor size.
Opportunity:
The researchers are looking for partners to take this invention to the next stage, which would be doing experiments to confirm the biological target, mode of action, and refine efficacy in vivo.
Photo activated cancer prodrug metallodrugs, light activation, drug delivery, prodrugs, pharmaceutical industry, biotech/medical companies
metallodrugs, light activation, drug delivery, prodrugs, pharmaceutical industry, biotech/medical companies
Photo activated cancer prodrug
Technology Overview:
Researchers at Leiden University in collaboration with researchers from Texas State University have developed a novel photo activated anticancer prodrug. This prodrug consists in a rigidin analogue caged by a ruthenium polypyridyl complex that can be released upon green light irradiation. The photocaged rigidin inhibitor is a microtubule polymerization inhibitor, and as such disrupts the formation of tumor vasculature.
Microtubule-targeting agents have been used in clinic for over 50 years, but they can be very toxic for the patient. The successful caging and release upon irradiation of the inhibitor means that it is now possible to keep the systemic concentration of the (pro)drug at a higher level than the level of toxicity, without such a high concentration causing significant biological effects. By shining light at the tumour site, a high dose of the inhibitor is only released locally (or in other words, the caged inhibitor is “hidden” in the dark).
Because the agent activation is irreversible, activity will be retained after light irradiation has been removed. Also, unlike in photodynamic therapy, where oxygen concentration in the irradiated tumor should be high enough, these new compounds remain active under hypoxic conditions (1% oxygen) as their activation mechanism does not rely on the presence of dioxygen.
Finally, attachment of the ruthenium-based caging significantly increases the water solubility of the drug in the dark.
Potential applications:
- Cancer therapy
State of development:
The 7-deazahypoxanthine synthetic analogues of marine alkaloid rigidins have shown promising anticancer activities; they were found to be highly effective in eradicating cancer cells in cell cultures at low doses (double- to single-digit nanomolar antiproliferative IC50 values) and showed statistically significant tumor size reduction in a colon cancer mouse model at nontoxic concentrations.
Opportunity:
The researchers are looking for partners from industry to take this invention to the next stage, which would be to carry out extensive in vivo (mouse model) studies.
IP Status:
The rigidin analogues have been patented by New Mexico Tech and Texas State University jointly, while a patent application has been filed for the caging technology by Texas State University and Leiden University jointly.
Reconstructed Human Skin Models for Research and Screening Purposes Four human skin equivalents that can be used for predictive screening or contract research that requires the complexity of human skin.
Four human skin equivalents that can be used for predictive screening or contract research that requires the complexity of human skin.
Reconstructed Human Skin Models for Research and Screening Purposes
Background
Functional 3D reconstructed human skin equivalents (HSEs) can be used for drug testing that avoids the excessive use of experimental animals. HSEs are three-dimensional systems that recapitulate most of the in vivo characteristics and in which cellular processes may be normalized compared to conventional monolayer cultures. These in vitro 3D HSEs are the result of more than 25 years of research and development by scientists from Leiden University Medical Center (LUMC) (The Netherlands). Within the Department of Dermatology, LUMC provide both healthy and diseased in vitro HSEs for animal-free compound screening and safety testing services and for co-development activities. HSEs are used in new product development and substantiation or product claims in cosmetic, pharmaceutical, food and environmental industries, but also in fundamental research within the field of experimental dermatology. These HSEs enable genomic, proteomic and drug development research not possible with traditional, unrepresentative or equally unavailable animal models, monolayer cell cultures or clinical test procedures. Uniquely, all in vitro HSEs are fully customizable to meet both the scientific and commercial demands of the customer.
Technology Overview
Currently LUMC offer four different HSEs; the Leiden Epidermal model (LEM), the Full-Thickness model (FTM), the Fibroblasts-Derived Matrix model (FDM) and the Ex-vivo human skin (ExHs) (Figure 1).
1. LEM consists of keratinocytes seeded on a non-cellular matrix (e.g. inert filter membrane or de-epidermized dermis. These epidermal models are suitable for e.g. skin toxicity, irritation, or penetration tests. In 2008, the researchers pre-validated the Leiden epidermal model (LEM) for skin irritation and corrosion (El Ghalbzouri et al., 2008).
2 FTM consists of keratinocytes, melanocytes and a fibroblast-populated three-dimensional collagen matrix. This model closely resembles native human skin. This full-thickness skin model can be used for tests, predictive screening and research on for example wound healing that requires the complexity of human skin, i.e. where the interaction between epidermal and dermal cells is crucial.
3. FDM is similar to the FTM model, but the dermal compartment consists of human fibroblast-derived extracellular matrix. This model can be used as a tool to evaluate the effect of e.g. ingredients on dermal processes in skin aging.
4. By using intact fresh skin biopsies, the researchers can perform short-term studies on human skin. By placing these skin samples onto an inert filter they can culture these ex vivo skin models (ExHs) up to 1 week.

(Figure 1.)
All models can be used for predictive screening or contract research that requires the complexity of human skin. Variations of these models can be generated by incorporation of other cell types (e.g. melanocytes, different types of fibroblasts, tumour cells etc.). The different models can also be grown at different oxygen concentrations.
Within the Department of Dermatology, LUMC have exploited the HSEs for more than ten years as a tool to conduct contract research for a number or large and medium size enterprises. These projects are focused on different aspects of healthy and diseased skin, such as; skin biology, dermal interactions, skin aging, wound healing, scars, treatment of bacterial infections by antibiotics and/or anti-microbial peptides. Some examples are given (Figure 2).

(Figure 2.)
Applications
There is an unmet clinical need for dermatology to develop therapies for a large number of skin diseases, such as skin cancer (eg melanoma), wound healing, bacterial wound infections, and eczema. There are currently no suitable in vitro models to screen and validate novel targets, and test the effects of potential new therapies in vitro . Cosmetics, Food, Chemical, Pharmaceutical, Medical devices.
Opportunity
The models are available for contract research and for co-development activities.
Keywords: dermatology, skin, keratinocytes, fibroblasts, melanocytes, wound healing, infection, eczema, cancer, penetration, irritation
Adjuvant Compounds for Conjugated Synthetic Vaccines Compounds which retain their strong adjuvanticity upon conjugation but possess much improved physicochemical properties
Compounds which retain their strong adjuvanticity upon conjugation but possess much improved physicochemical properties
Adjuvant Compounds for Conjugated Synthetic Vaccines
Background
Synthetic vaccines consist of well-defined chemically produced molecules and require two components: an antigen and an immune-stimulating adjuvant. In peptide-based synthetic vaccines the antigen is a peptide that contains a sequence (the epitope) that is specifically recognized by the T cell immune system while the adjuvant is often a ligand of one of Toll-like receptors (TLR’s) that provides a “danger signal” and expands specific T cells. In this invention an improved adjuvant is described that can be used as the entity covalently attached to any synthetic peptide sequence.
Technology Overview
This technology builds on a well-known design of conjugated vaccines comprising synthetic lipopeptides, in which the lipophilic TLR-2 agonist is attached to the N-terminus of synthetic antigenic peptide. Researchers from Leiden University have previously shown that classical TLR2 ligand-conjugated peptide vaccines has strong T cell activation potency in vivo and can control aggressive tumor growth. The compound contains a defined lipid-component which can be covalently attached to any synthetic peptide sequence. The adjuvant compound retains its strong adjuvanticity upon conjugation but possesses much improved physicochemical properties as compared to established lipid-based synthetic vaccines. The synthesis of the conjugatable TLR-2 agonist is accomplished by the methods of solution phase organic chemistry and subsequently it is readily coupled to a peptide sequence pre-assembled on solid-phase by standard method of peptide chemistry. The finished conjugate is liberated from the solid-phase and purified by the techniques common in the preparation of peptide-based vaccines. The conjugable TLR2 lipopeptide adjuvant platform is suited to augment the immunogenicity of synthetic peptide-based vaccines to any pathogen or malignancy of which sequence information of immunogenic epitopes is available. Especially for personalized vaccines for cancer patients this adjuvant offers a flexible system to swiftly conjugate multiple peptide sequences of tumor-specific mutated neo-epitopes.
Details and State of Development:
A solution synthesis of a conjugatable derivative of the TLR-2 agonist on gram-scale is developed as well as a general approach to the solid-phase synthesis of the lipopeptides connected to this adjuvant. Immunological characterization of the TLR-2 agonist has shown:
- Strong TLR2 activation of reporter cell lines;
- Functional maturation of human dendritic cell cultures; and
- Improvement of antigen presentation of conjugated synthetic peptide harboring neo-epitope sequence to melanoma patient-derived human T cells
Applications
1. Peptide based vaccines
2. Personalized vaccines
Keywords: adjuvant, agonist, cell, activation, immune response, immunological ligands, synthetic vaccine
Platinum Metal-Based Anticancer and Antibacterial Drugs Novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents
Novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents
Platinum Metal-Based Anticancer and Antibacterial Drugs
Technology Overview
Researchers at Leiden University have developed novel palladium, platinum, and gold compounds that may be used as anticancer or antibacterial agents. A family of related ligands can be used in the formulation of the compounds, to tune solubility and activity. The compounds are very stable due to the tetradentate nature of the ligands, coupled with a tetracoordinated (d8) metal center.
While these novel compounds are both simple and cheap to synthesize, they could provide an interesting new alternative for platinum-based anticancer drugs (platinum based drugs such as cisplatin, oxaliplatin and carboplatin already have FDA-approval and are used widely). These new compounds are all the more interesting, because micromolar to nanomolar activities have been observed on cancer cell lines that are resistant to cisplatin.
However the activities are not limited to cancer therapy; applications as an antibacterial agent are also possible. The exact activities of the different compounds depend on the entire compound, while the mode of action depends critically on the nature of the metal.
Details and State of Development:
- The compounds have been synthesized and characterized. In vitro and in vivo (mouse model) experiments have been conducted that have confirmed the high potential of the compounds; in vitro experiments have revealed that the cytotoxicity of some derivatives on human cancer cell lines are among the highest ever reported in the literature, while preliminary in vivo experiments in a mouse model show a decrease in tumor size.
Applications
1. Cancer therapy
2. Antibacterial agent
Opportunity
The researchers are looking for partners to take this invention to the next stage, which would be doing experiments to confirm the biological target, mode of action, and refine efficacy in vivo.
Keywords: metallodrugs, bacteria, cancer, pharmaceutical industry, biotech/medical companies
Photoactivatable Anticancer Prodrug A novel photo activated anticancer prodrug consisting in a rigidin analogue caged by a ruthenium polypyridyl complex
A novel photo activated anticancer prodrug consisting in a rigidin analogue caged by a ruthenium polypyridyl complex
Photoactivatable Anticancer Prodrug
Background
In oncology non-resectable, non-metastasized tumor treatment currently relies on chemotherapy, radiation therapy, or photodynamic therapy (PDT). Chemotherapy can be effective but it can be done because of side effects (neuron damage, pain, fatigue, etc.). Radiation therapy and PDT are more local and lower systemic toxicity but they require dioxygen in the irradiated tissues to be efficient. Hypoxic tumors, subset or tumors with high volume or hypoxic, poorly vascularized tissues, low prognosis for the patient.
The present invention is a form of photoactivated chemotherapy (PACT) that combines the advantages of well-defined target (PDT) and PDT (local activation by light and lower side effects). It is more specifically aimed at treating hypoxic tumors.
Technology Overview
The technology relies on the photochemical breakage of a chemical bond. In the prodrug form) a toxic, thioether-containing microtubule polymerization inhibitor is coordinated to a non-toxic ruthenium (II) caging complex. In the dark, the coordination bond between Ru 2+ and the sulfur atom is stable, but under light irradiation is broken, and the non-toxic caging group.
Details and State of Development:
- Collaboration between Leiden University, NL, and Texas State University, USA.
- Uncaged microtubule inhibitor patented by Prof. Alexander Kornienko from Texas State University, USA
- Synthesis, photochemistry, dark stability, and microtubule polymerization inhibition upon light irradiation, have been demonstrated
Chromatography-free synthesis available
- Low dark toxicity and high toxicity in vitro in 2D human cancer cell monolayers demonstrated both in normoxic (21% O 2 ) and hypoxic (1% O 2 ) conditions
- Low dark toxicity and high toxicity in vitro in A549 lung cancer 3D tumor spheroids
- Preliminary results in vivodemonstrate 30% tumor volume reduction under green light irradiation in A549 lung tumor xenografts in nude mice, and no toxicity in the dark, after intraperitoneal injection at 1 mg / kg and with a green light dose of 38 J / cm 2 .
Applications
Market for photoactivated chemotherapy includes brain, liver, head and neck, non-melanoma skin, and eye cancer (Market study available). Best application for tumors with a high ratio of hypoxic to normoxic volume, for which currently available therapies (PDT, radiation, surgery) do not work or are impossible (non-resectable tumors).
Opportunity
The researchers are looking for partners in the field of research, which would be extensive in vivo (mouse model) studies.
Liposome Drug Delivery Vector Targeting the Blood Brain Barrier A lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle
A lipid, which when mixed with a naturally occurring phospholipid and formulated into 100 nm liposomes, results in a drug delivery vehicle
Liposome Drug Delivery Vector Targeting the Blood Brain Barrier
Background
In terms of drug delivery, the BBB is a formidable barrier. Current (pre-clinical) state-of-the-art drug delivery systems, designed to specifically target the BBB, maximally deliver <0.5% of the total injected drug dose to the brain. As such, prognoses for diseases of the brain (eg. Alzheimer’s disease, glioblastoma) remain notoriously poor.
Technology Overview
Researchers at Leiden University have developed a novel lipid, which when mixed with a naturally occurring phospholipid and formulated into 150 nm liposomes, results in a drug delivery vehicle with >10-fold selectivity for the brain endothelium (ie. the BBB) over the systemic endothelium. Encapsulation of both small molecule drugs (doxorubicin) and inorganic nanoparticles (gold nanoparticles) has been successfully demonstrated, resulting in the selective delivery of these reagents to the BBB (following intravenous injection). The novel lipid, required for BBB targeting, is synthesised in a single synthetic step (plus purification) using readily available reagents (<10 Euro/g, Sigma).
Details and State of Development:
The researchers have also demonstrated proof-of-principle of successful encapsulation of small molecule drugs as well as larger cargoes in the newly developed nanocarrier.
Applications
1. Drugs specifically targeting the brain and/or the BBB, such as treatments for strokes, cancer and neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, Huntington’s).
2. Enhancement of brain and/or BBB (theranostic) imaging.
Opportunity
All results have so far been confirmed in the embryonic zebrafish. Validation of this is technology in rodent models is currently ongoing. The researchers are looking for partners from industry/academia/SME to take this invention to the next stage: in vivo testing in mammalian models for brain-related diseas
Keywords: pharmaceutical industry, biotech/medical companies, neuropharma, drug delivery platform
High Throughput 3D Cell Culture Assay An automated method to create 3D ECM-embedded cell spheroids that overcomes previously identified limitations
An automated method to create 3D ECM-embedded cell spheroids that overcomes previously identified limitations
High Throughput 3D Cell Culture Assay
Background
The need for better in vitro screening technology is well known and documented in the field. The currently used 2D cell assays are fast and easy to perform, but lack predictive value. Among the various 3D culture platforms, 3D ECM-embedded cell spheroids most closely represent the in vivo tumor microenvironments. However, several technical hurdles preclude the use of such cultures in high content screening (HCS): i) spheroid creation is difficult and poorly reproducible: ii) it is restricted to certain cell types and iii) spheroid location cannot be carefully controlled, which hampers automated imaging.
Technology Overview
Scientists at Leiden University have developed an automated method to create 3D ECM-embedded cell spheroids that overcomes these limitations (Figure 1). Spheroid formation time is strongly reduced compared to other methods (minutes rather than days) and it can be applied to a broad range of cell types including cells that naturally do not form cell-cell contacts, endothelial cells, various cancer cell lines, and primary tumor cell suspensions. For High Content Screening, they are able to produce 1 spheroid per well in 384 well plates or up to 7 patterned spheroids per well in 96 well plates. Importantly, the spheroids have defined xyz spatial coordinates allowing automated confocal imaging and image analysis algorithms.

Details and State of Development:
- Proof of principle has been established.
Applications
1. Cell culture
2. Tissue engineering
3. High-throughput drug screening
4. Cancer / tumor research
Keywords: 3D cell culture, cell spheroids, high-throughput, drug screening, breast cancer, cancer
New Fixatives with 15-Fold Lower Formaldehyde Concentration Two low-hazardous alternatives to formalin 10% with 10- to 15-fold lower formaldehyde concentration and no change to your histopathology protocol.
Two low-hazardous alternatives to formalin 10% with 10- to 15-fold lower formaldehyde concentration and no change to your histopathology protocol.
New Fixatives with 15-Fold Lower Formaldehyde Concentration
Background
Formalin has stood as the 'gold standard' fixative in pathology labs largely due to its high degree of accuracy, compatibility with downstream histological applications, low cost and ease of use.
Nevertheless, due to the associated health hazards of formaldehyde (1B carcinogen IARC), the search for formalin replacements has been on-going for years, further motivated by the Occupational Safety and Health Administration (OSHA), which encourages its replacement despite the listed benefits . The most well-known alternatives are alcohol-based fixatives, mostly based on ethanol or methanol at concentrations of 50% and higher. However, alcohols do not preserve tissue morphology as well as formaldehyde-based fixatives, the solutions are highly flammable, (neuro) toxic and ethanol possesses the same group 1 carcinogen classification as formaldehyde by the International Agency for Research on Cancer (IARC).
Technology Overview
Leiden University Medical Center and Fix for Life BV have developed two low - hazardous fixatives which require no histopathology protocol adjustments (identical to neutral buffered formalin 10% protocol) and benefit from 10‑ to 15-fold lower concentration of hazardous formaldehyde and better yields and quality of extracted cDNA and RNA. Further PCR experiments are ongoing.
Application
Histopathology fixative for tissue samples
Tumor Associated Carbohydrate Antigens as Targets for Colorectal Cancer Immunotherapy The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue.
The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue.
Tumor Associated Carbohydrate Antigens as Targets for Colorectal Cancer Immunotherapy

Summary
The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue. Furthermore, it is believed that the markers disclosed may be useful therapeutic targets easily accessible as expressed on cell surfaces directly accessible to therapeutics and can be carried by multiple
proteins, reflecting the overall glycosylation phenotype of the cell, providing a broader tumor targeting strategy.
There was a patent filed recently which covers the workflow of extracting, identifying and analysing these highly specific structures as well as another one that includes the specific TACA structures which were found to be solely present in CRC and not in healthy colon providing a number of promising targets for potential therapy.
Background
Colorectal cancer (CRC) is one of the leading malignancies worldwide with over 900,000 deaths in 20201. Conventional therapeutic strategies include chemotherapy, radiation therapy, and surgery. However, due to poor screening strategies and lack of symptoms in early stages, most cases are detected at an advanced stage, leading to unsuccessful treatment. Unraveling the glycome in cancer is important for the development of immunotherapies for treating solid tumors and the discovery of cancer-specific glycan structures is critical for improving how these cancers are targeted.
Technology
The present invention provides a better understanding of the variation in
O-glycosylation and its association with cell phenotypes. An in-depth
structural O-glycosylation analysis of 26 CRC cell lines was performed.
Additionally, the O-glycosylation signatures of primary cell lines as well as the primary and metastatic colorectal cancers they have been derived from were mapped and compared. Furthermore, glycosylation signatures of paired micro-dissected cancer and healthy mucosa were investigated. The released O-glycans were analysed on a sensitive nano-liquid chromatography coupled to a tandem mass spectrometer using electrospray ionization enabling powerful separation of isomeric species, as well as in-depth structural characterization of the epitopes. The major outcomes were as follows:
- Using transformative technology based on laser capture microdissection allowed for the first time to dissect the cancer cell O-glycome of CRC.
- Highly specific glycan structures were observed in cancer cells from most of the tumor tissue which were not present in healthy control tissue.
Value Proposition
The global colorectal cancer therapeutics market is poised to grow by USD 994.94 million during 2019-2023, progressing at a CAGR of almost 3% (www.businesswire.com).
Team
Prof Manfred Wuhrer currently holds the position as Head of the Center for Proteomics and Metabolomics (CPM) at the Leiden University Medical
Center in Leiden/ The Netherlands. Dr Guinivere Lageveen Kammeijer is
Senior Scientist at the CPM focusing on exploring cancer glycosylation for developing novel therapeutic and diagnostic applications. Dr Katarina
Madunic is Scientist at the CPM specialized in dissecting cancer cell
glycosylation.
Cytotoxicity of Synthesized EPD (EPD-S), A Natural Sesquiterpene Lacton, and its Future Clinical Efficacy The present invention is relating to a new anti-cancer drug, EPD-S. This drug shows on itself, as well as in combination with paclitaxel and cisplatin, strong cytotoxicity against ovarian cancer and other cancer types.
The present invention is relating to a new anti-cancer drug, EPD-S. This drug shows on itself, as well as in combination with paclitaxel and cisplatin, strong cytotoxicity against ovarian cancer and other cancer types.
Cytotoxicity of Synthesized EPD (EPD-S), A Natural Sesquiterpene Lacton, and its Future Clinical Efficacy
Background
Ovarian cancer remains still the leading cause of death of gynecological malignancy, in spite of first-line chemotherapy with paclitaxel and cisplatin. Although initial a favorable response, relapses are common and prognosis stays poor.
A plant, Calomeria amaranthoides of the family Asteraceae, endemic to Australia, has been collected in the Blue Mountains (NSW). Asteraceae are known for their natural active compounds, including sesquiterpene lactones (SL’s) which are known for their potential as anti-cancer agents.
This new anti-cancer drug, Eremophilanolides-1-(10)-11(13)-dien-12,8β-olide or EPD, has been proven a very interesting anti-cancer drug. It has exhibited potent cytotoxic effects towards ovarian cancer and other cancers, like melanoma, sarcoma, colon, thyroid, leukemia, breast, but not towards normal cells.
Technology
Most of the experiments have been performed “in vitro”, using the IC50
tests on ovarian cancer cell lines, but also on other cancer cell lines. A
study with nude mice showed that EPD did better than cisplatin, used as
a positive control.
Since EPD has been synthesized by Syncom, (Groningen) (EPD-S) it hasbeen studied by Oncolines and Oncolines Profiler by The Netherlands Translational Research Center (NTRC, Oss, The Netherlands).
Not only has EPD-S been proven cytotoxic on its own, it also has shown strong synergism with paclitaxel and cisplatin in resistant ovarian cancer
cell lines and other cancer cell lines. EPD, in combination with cisplatin, was studied in cell lines with a BRCA1 mutation. In comparison with
olaparib (medication for patients with BRCA1 mutation), showed EPD-S
much stronger chemosensitivity. EPD –S is blocking the pathway of NF-kβ, with apoptosis as result.
Value Proposition
The global Ovarian Cancer market size alone is expected to gain market
growth in the forecast period of 2020 to 2025, with a CAGR of 9.0% in the forecast period of 2020 to 2025 and will expected to reach USD 2382
million by 2025, from USD 1684.4 million in 2019 (https://www.marketwatch.com/).
Team
Dr Caroline van Haaften is the sole inventor of the technology, and she
currently holds the position as Head of Carocell Nederland B.V.
Targeting ligand-bound (solid-phase) C1q We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q.
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q.
Targeting ligand-bound (solid-phase) C1q

SUMMARY
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q. This property provides unique opportunities as now these antibodies can be used to target C1q in immune complexes or C1q in specific tissues without interference of circulating C1q. Binding to circulating C1q would not only cause a huge sink for any
therapeutics or tracers, but it would also interfere with the normal C1q functions, cause clearance of C1q and reduced complement activity. Using t
BACKGROUND
C1q is the recognition molecule of the classical pathway of complement activation. C1q circulates in the blood at a concentration of around 150 μg/ml. C1q only acquires the capacity to activate the complement system after binding to an array of its ligands. These ligands include for example, immune complexes comprising antigen bound IgG antibodies or antigen bound IgM as well as ligand bound C-reactive protein. If these ligands are present in a sufficiently multimeric format than C1q binds, which results in activation of the C1 enzymes, C1r and C1s and subsequent complement activation takes place. These processes are beneficial in fighting infections and in fighting tumors, but unfortunately do also occur on host tissues where e.g. autoantibodies accumulate. Blocking C1q and classical pathway activity by antibodies has been performed, it depletes all circulating C1q and blocks classical pathway activity, however this works systemically and may put the patient in danger of infections and development of autoimmunity. Here we describe the use of very different anti-C1q autoantibodies, now targeting only ligand-bound C1q while not binding to / interfering with circulating C1q. Such anti-C1q autoantibodies have been described, also by our team, to occur in lupus patients and in some controls. They were shown to be pathogenic, but only if ligand-bound C1q was present in target organs. Obviously, the developed antibodies will be engineered to acquire the desired immune effector properties. Here we describe new, completely human antibodies that we produce recombinantly. This allows us to modify the Fc-domain of the antibody in such a way that it can no longer bind Fc-Receptors or trigger additional complement activity. In this format the antibodies can be safely used as tracers or as antibody drug conjugates. In conditions where one would like to amplify inflammation we can introduce mutations that will enhance Fc-Receptor interactions, that will boost complement activity or that may engage specific immune cells to fight cancer or (antibiotic resistant) infections.
TECHNOLOGY
The invention builds on the use of antibodies that bind to solid-phase C1q. Combined with available technologies we can generate antibodies with inert or active Fc-domains regarding immune activation. In addition we can generate tracers, and antibody-drug conjugates.
VALUE PROPOSITION
The global complement-targeted therapeutics market is expected to
grow at a CAGR of 10.5% from 2022 to 2030. The growth in this
market can be attributed to the increasing prevalence of complement-mediated diseases, rising demand for novel therapies, and growing investments in R&D by pharmaceutical companies.
TEAM
Dr. Leendert Trouw currently holds a position as Professor at the
Department of Immunology at the Leiden University Medical Center
in Leiden, The Netherlands.

